Interview with Charles A. Barnes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tribute to Valentine Telegdi
Tribute to Valentine Telegdi who passed away on April 8th in Pasadena by K. Freudenreich, ETHZ/IPP/LHP Plenary CHIPP Meeting, PSI, October 2, 2006 Val Telegdi: years 1922 - 1943 Born on January 11th, 1922 in Budapest, spent only a few years in Hungary. According to his own words in his younger years he was a master of “involuntary tourism” , participating passively in German occupations in three countries: Austria, Belgium and Northern Italy. He attended grammar school in Vienna and then a technical school in Brussels. From 1940 - 1943 he worked in a patent attorney’s office in Milan. He used to say that - contrary to Albert Einstein in Berne - being on the other side of the fence he really had to work hard. When the Germans occupied Northern Italy Val, together with his mother, fled to Switzerland. October 2, 2006, Plenary CHIPP Meeting, PSI, K. Freudenreich, ETHZ 1/20 Val Telegdi: years 1943 - 1946 After a short internment in a refugee camp he joined his father in Lausanne where he studied chemical engineering at the EPUL with a grant from the F onds Europ´een de Secours aux Etudiants. At the EPUL he also attended lectures in theoretical physics given by E.C.G. Stuckelberg¨ von Breidenbach whom he estimated very highly. Ironic telegram by Gell-Mann to “congratulate” Feynman for his Nobel prize: “Now you can give back my notes”, signed Stuckelberg¨ Stuckelberg¨ helped Val to be accepted by P. Scherrer at ETHZ. October 2, 2006, Plenary CHIPP Meeting, PSI, K. Freudenreich, ETHZ 2/20 Val Telegdi: years 1946 - 1951 In 1946 the institute of physics was located at the Gloriastrasse. -

RHIC Begins Smashing Nuclei
NEWS RHIC begins smashing nuclei Gold at STAR - side view of a collision of two 30 GeV/nucleon gold End view in the STAR detector of the same collision looking along beams in the STAR detector at the Relativistic Heavy Ion Collider at the direction of the colliding beams. Approximately 1000 tracks Brookhaven. were recorded in this event On Monday 12 June a new high-energy laboratory director for RHIC. It was a proud rings filled, the ions will be whipped to machine made its stage debut as operators in moment for Ozaki, who returned to 70 GeV/nucleon. With stable beams coasting the main control room of Brookhaven's Brookhaven from Japan to oversee the con around the rings, the nuclei collide head-on, Relativistic Heavy Ion Collider (RHIC) finally struction and commissioning of this eventually at the rate of tens of thousands of declared victory over their stubborn beams. challenging machine. collisions per second. Several weeks before, Derek Lowenstein, The high temperatures and densities Principal RHIC components were manufac chairman of the laboratory's collider-acceler achieved in the RHIC collisions should, for a tured by industry, in some cases through ator department, had described repeated fleeting moment, allow the quarks and gluons co-operative ventures that transferred tech attempts to get stable beams of gold ions to roam in a soup-like plasma - a state of nology developed at Brookhaven to private circulating in RHIC's two 3.8 km rings as "like matter that is believed to have last existed industry. learning to drive at the Indy 500!". -

An Improbable Venture
AN IMPROBABLE VENTURE A HISTORY OF THE UNIVERSITY OF CALIFORNIA, SAN DIEGO NANCY SCOTT ANDERSON THE UCSD PRESS LA JOLLA, CALIFORNIA © 1993 by The Regents of the University of California and Nancy Scott Anderson All rights reserved. Library of Congress Cataloging in Publication Data Anderson, Nancy Scott. An improbable venture: a history of the University of California, San Diego/ Nancy Scott Anderson 302 p. (not including index) Includes bibliographical references (p. 263-302) and index 1. University of California, San Diego—History. 2. Universities and colleges—California—San Diego. I. University of California, San Diego LD781.S2A65 1993 93-61345 Text typeset in 10/14 pt. Goudy by Prepress Services, University of California, San Diego. Printed and bound by Graphics and Reproduction Services, University of California, San Diego. Cover designed by the Publications Office of University Communications, University of California, San Diego. CONTENTS Foreword.................................................................................................................i Preface.........................................................................................................................v Introduction: The Model and Its Mechanism ............................................................... 1 Chapter One: Ocean Origins ...................................................................................... 15 Chapter Two: A Cathedral on a Bluff ......................................................................... 37 Chapter Three: -

Communications-Mathematics and Applied Mathematics/Download/8110
A Mathematician's Journey to the Edge of the Universe "The only true wisdom is in knowing you know nothing." ― Socrates Manjunath.R #16/1, 8th Main Road, Shivanagar, Rajajinagar, Bangalore560010, Karnataka, India *Corresponding Author Email: [email protected] *Website: http://www.myw3schools.com/ A Mathematician's Journey to the Edge of the Universe What’s the Ultimate Question? Since the dawn of the history of science from Copernicus (who took the details of Ptolemy, and found a way to look at the same construction from a slightly different perspective and discover that the Earth is not the center of the universe) and Galileo to the present, we (a hoard of talking monkeys who's consciousness is from a collection of connected neurons − hammering away on typewriters and by pure chance eventually ranging the values for the (fundamental) numbers that would allow the development of any form of intelligent life) have gazed at the stars and attempted to chart the heavens and still discovering the fundamental laws of nature often get asked: What is Dark Matter? ... What is Dark Energy? ... What Came Before the Big Bang? ... What's Inside a Black Hole? ... Will the universe continue expanding? Will it just stop or even begin to contract? Are We Alone? Beginning at Stonehenge and ending with the current crisis in String Theory, the story of this eternal question to uncover the mysteries of the universe describes a narrative that includes some of the greatest discoveries of all time and leading personalities, including Aristotle, Johannes Kepler, and Isaac Newton, and the rise to the modern era of Einstein, Eddington, and Hawking. -

In the PHYSICAL SCIENCES
UNITED STATES ATOMIC ENERGY COMMISSION RESEARCH CONTRACTS in the PHYSICAL SCIENCES he JULY 1, 1969 DIVISION OF RESEARCH INDEX Page Introduction .................................. ....................... ......... 2 List of Federally Funded Research and Development Centers ..................... 5 Summary of Off-Site Contracts ................................................. 6 Summary of New Proposals Received and Actions Taken ........................... 7 Summary of Contracts by State ................................................. 8 Contract Listing: High Energy Physics .................................................. 13 Medium Energy Physics ................................................ 15 Low Energy Physics ................................................... 16 Mathematics and Computer Research .................................... 20 Chemistry ......... .............................................. 22 Metallurgy and Materials ............................................. 32 Controlled Thermonuclear Research .................................... 41 1 INTRODUCT ION The Physical Research Program is chiefly concerned with basic research investigations undertaken to discover new scientific knowledge and also includes some applied research investigations relevant to certain aspects of the practical utilization of nuclear energy. Research is conducted in the fields of high, medium, and low energy physics, mathematics and computers, chemistry, metallurgy and materials, and controlled thermonuclear reactions. Approximately three-fourths -

Center for History of Physics Newsletter, Spring 2008
One Physics Ellipse, College Park, MD 20740-3843, CENTER FOR HISTORY OF PHYSICS NIELS BOHR LIBRARY & ARCHIVES Tel. 301-209-3165 Vol. XL, Number 1 Spring 2008 AAS Working Group Acts to Preserve Astronomical Heritage By Stephen McCluskey mong the physical sciences, astronomy has a long tradition A of constructing centers of teaching and research–in a word, observatories. The heritage of these centers survives in their physical structures and instruments; in the scientific data recorded in their observing logs, photographic plates, and instrumental records of various kinds; and more commonly in the published and unpublished records of astronomers and of the observatories at which they worked. These records have continuing value for both historical and scientific research. In January 2007 the American Astronomical Society (AAS) formed a working group to develop and disseminate procedures, criteria, and priorities for identifying, designating, and preserving structures, instruments, and records so that they will continue to be available for astronomical and historical research, for the teaching of astronomy, and for outreach to the general public. The scope of this charge is quite broad, encompassing astronomical structures ranging from archaeoastronomical sites to modern observatories; papers of individual astronomers, observatories and professional journals; observing records; and astronomical instruments themselves. Reflecting this wide scope, the members of the working group include historians of astronomy, practicing astronomers and observatory directors, and specialists Oak Ridge National Laboratory; Santa encounters tight security during in astronomical instruments, archives, and archaeology. a wartime visit to Oak Ridge. Many more images recently donated by the Digital Photo Archive, Department of Energy appear on page 13 and The first item on the working group’s agenda was to determine through out this newsletter. -

Trinity Transcript
THE NATIONAL ACADEMIES Committee on International Security and Arms Control 60th Anniversary of Trinity: First Manmade Nuclear Explosion, July 16, 1945 PUBLIC SYMPOSIUM July 14, 2005 National Academy of Sciences Auditorium 2100 C Street, NW Washington, DC Proceedings By: CASET Associates, Ltd. 10201 Lee Highway, Suite 180 Fairfax, VA 22030 (703) 352-0091 CONTENTS PAGE Introductory Remarks Welcome: Ralph Cicerone, President, The National Academies (NAS) 1 Introduction: Raymond Jeanloz, Chair, Committee on International Security and Arms Control (CISAC) 3 Roundtable Discussion by Trinity Veterans Introduction: Wolfgang Panofsky, Chair 5 Individual Statements by Trinity Veterans: Harold Agnew 10 Hugh Bradner 13 Robert Christy 16 Val Fitch 20 Don Hornig 24 Lawrence Johnston 29 Arnold Kramish 31 Louis Rosen 35 Maurice Shapiro 38 Rubby Sherr 41 Harold Agnew (continued) 43 1 PROCEEDINGS 8:45 AM DR. JEANLOZ: My name is Raymond Jeanloz, and I am the Chair of the Committee on International Security and Arms Control that organized this morning’s symposium, recognizing the 60th anniversary of Trinity, the first manmade nuclear explosion. I will be the moderator for today’s event, and primarily will try to stay out of the way because we have many truly distinguished and notable speakers. In order to allow them the maximum amount of time, I will only give brief introductions and ask that you please turn to the biographical information that has been provided to you. To start with, it is my special honor to introduce Ralph Cicerone, the President of the National Academy of Sciences, who will open our meeting with introductory remarks. He is a distinguished researcher and scientific leader, recently serving as Chancellor of the University of California at Irvine, and his work in the area of climate change and pollution has had an important impact on policy. -

History Newsletter CENTER for HISTORY of PHYSICS&NIELS BOHR LIBRARY & ARCHIVES Vol
History Newsletter CENTER FOR HISTORY OF PHYSICS&NIELS BOHR LIBRARY & ARCHIVES Vol. 46, No. 2 • Fall 2014 The History Programs Publish Physics Entrepreneurship and Innovation By R. Joseph Anderson, Director, Niels Bohr Library & Archives We completed our most recent study PhD physicists and other professionals energy sources, and laser sensors and of physicists working in the private who co-founded and work at some 91 communications, along with a variety of sector at the end of 2013, and the startup companies in 14 states that were new manufacturing tools. final report, Physics Entrepreneurship established in the last few decades. and Innovation, is now available The four-year study is focused on both in print and online. While the investigating the structure and physicists in the study don’t fit the dynamics of physics entrepreneurship conventional model of hard-driving, and understanding some of the risk taking entrepreneurs, physics- factors that lead to the success or based entrepreneurship plays a vital failure of new startups, including role in innovation and the ongoing funding, technology transfer, location, transformation of American industry business models, and marketing. We in just about every business sector. have also considered ways that the companies can work with private For much of the 20th century, and public archives to preserve technological innovations that drove historically valuable records so that U.S. economic growth emerged from future researchers can understand "idea factories" housed within large today’s technology and economy. Our companies—research units like Bell findings include: Labs or Xerox PARC that developed everything from the transistor to • No national standard of entrepre- the computer mouse. -
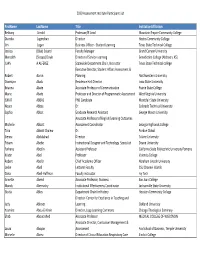
2020 Assessment Institute Participant List Firstname Lastname Title
2020 Assessment Institute Participant List FirstName LastName Title InstitutionAffiliation Bethany Arnold Professor/IE Lead Mountain Empire Community College Diandra Jugmohan Director Hostos Community College Jim Logan Business Officer ‐ Student Learning Texas State Technical College Jessica (Blair) Soland Faculty Manager Grand Canyon University Meredith (Stoops) Doyle Director of Service‐Learning Benedictine College (Atchison, KS) JUAN A ALFEREZ Statewide Department Chair, Instructor Texas State Technical college Executive Director, Student Affairs Assessment & Robert Aaron Planning Northwestern University Osomiyor Abalu Residence Hall Director Iowa State University Brianna Abate Associate Professor of Communication Prairie State College Marie Abate Professor and Director of Programmatic Assessment West Virginia University ISMAT ABBAS PhD Candidate Montclair State University Noura Abbas Dr. Colorado Technical University Sophia Abbot Graduate Research Assistant George Mason University Associate Professor of English/Learning Outcomes Michelle Abbott Assessment Coordinator Georgia Highlands College Talia Abbott Chalew Dr. Purdue Global Sienna Abdulahad Director Tulane University Fitsum Abebe Instructional Designer and Technology Specialist Doane University Farhana Abedin Assistant Professor California State Polytechnic University Pomona Kristin Abel Professor Valencia College Robert Abel Jr Chief Academic Officer Abraham Lincoln University Leslie Abell Lecturer Faculty CSU Channel Islands Dana Abell‐Huffman Faculty instructor Ivy Tech Annette -

Philanthropist Pledges $70 M to Homestake Underground Lab
CCESepFaces43-51 16/8/06 15:07 Page 43 FACES AND PLACES LABORATORIES Philanthropist pledges $70 m to Homestake Underground Lab South Dakota governor Mike Rounds (third from right) and philanthropist T Denny Sanford (fourth from right) prepare to cut the ribbon at the official dedication of the Sanford Underground Science and Engineering Laboratory in the former Homestake gold mine. At the official dedication of the Homestake 4200 m water equivalent). In November 2005 donation in South Dakota, including major Underground Laboratory on 26 June, South the Homestake Collaboration issued a call for contributions to a children’s hospital centred Dakota resident, banker and philanthropist letters of interest from scientific at the University of South Dakota, and other T Denny Sanford created a stir by pledging collaborations that were interested in using educational and child-oriented endeavours. $70 m to help develop the multidisciplinary the interim facility. The 85 letters received His gift expands the alliance supporting the laboratory in the former Homestake gold comprised 60% proposals from earth science Sanford Underground Science and mine. The mine is one of two finalists for the and 25% from physics, with the remainder for Engineering Laboratory at Homestake US National Science Foundation effort to engineering and other uses. (SUSEL), joining the State of South Dakota, establish a Deep Underground Science and The second installment of $20 m by 2009 the US National Science Foundation (NSF) Engineering Laboratory (DUSEL), which will be will create the Sanford Center for Science through its competitive site selection process, a national laboratory for underground Education – a 50 000 ft2 facility in the historic the Homestake Scientific Collaboration and experimentation in nuclear and particle mine buildings. -

Robert Dicke and the Naissance of Experimental Gravity Physics
Eur. Phys. J. H 42, 177–259 (2017) DOI: 10.1140/epjh/e2016-70034-0 THE EUROPEAN PHYSICAL JOURNAL H RobertDickeandthenaissance of experimental gravity physics, 1957–1967 Phillip James Edwin Peeblesa Joseph Henry Laboratories, Princeton University, Princeton NJ, USA Received 27 May 2016 / Received in final form 22 June 2016 Published online 6 October 2016 c The Author(s) 2016. This article is published with open access at Springerlink.com Abstract. The experimental study of gravity became much more active in the late 1950s, a change pronounced enough be termed the birth, or naissance, of experimental gravity physics. I present a review of devel- opments in this subject since 1915, through the broad range of new approaches that commenced in the late 1950s, and up to the transition of experimental gravity physics to what might be termed a normal and accepted part of physical science in the late 1960s. This review shows the importance of advances in technology, here as in all branches of nat- ural science. The role of contingency is illustrated by Robert Dicke’s decision in the mid-1950s to change directions in mid-career, to lead a research group dedicated to the experimental study of gravity. The re- view also shows the power of nonempirical evidence. Some in the 1950s felt that general relativity theory is so logically sound as to be scarcely worth the testing. But Dicke and others argued that a poorly tested theory is only that, and that other nonempirical arguments, based on Mach’s Principle and Dirac’s Large Numbers hypothesis, suggested it would be worth looking for a better theory of gravity. -

Fermilab- Uchicago Connections
Fermilab- UChicago Connections Pushpa Bhat (Fermilab) Fermi50 @ University of Chicago 31 October 2017 1 10/31/17 Pushpa Bhat | Fermi50 @ UChicago The National Accelerator Laboratory created in 1967, renamed Fermilab in 1974, opened a new frontier in the exploration of matter and energy. Given a rich tradition in physics and the legacy of legendary physicists … Michelson, Millikan, Compton,.. to Fermi and beyond, it should be no surprise that the University of Chicago would have strong influence on this frontier laboratory in the suburbs of Chicago. 2 10/31/17 Pushpa Bhat | Fermi50 @ UChicago Strong and sustained connections UChicago’s connections with Fermilab dates back to the beginning of the Lab: UChicago was one of the charter members of the Universities Research Association (URA) in 1967. The Atomic Energy Commission (AEC) mentions the proximity of many excellent universities in the mid-west including UChicago as one of the reasons to select the Weston site. George Beadle, the President of UChicago (1961-68) had a committee (1967) on relationships between UChicago and NAL. Throughout the past decades, many Fermilab scientists have had joint appointments with UChicago, including our first director, Bob Wilson. Faculty at UChicago have held high positions at the Lab and have influenced the research program at the Lab. 3 10/31/17 Pushpa Bhat | Fermi50 @ UChicago Transfer of Title for NAL site to AEC The State of Illinois purchased the 6800 acre Weston site in Dec. 1966 and transferred the title to AEC on April 10, 1969. The