Connective Tissue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

An Histologie and Immunohistochemical Study
Bull Group Int Rech Sci Stomatol et Odontol - Vol 34 n° 3-4, 1991 The connective tissue cells of human dental pulp: An histologie and immunohistochemical study LYN Peifen*, FIORE-DONNO G.** and LOMBARD1 T.** * Department ofStomatology, Tbird Hospital ofBeijing Medical University, China. ** Division of Stomatology School ofDental Medicine, Faculty ofMedicine Geneva, Switzerland. SUMMARY Twenty human healthy teeth were extracted for orthondontic purposes and processed for histological, and immunohistochemical examination. Odontoblasts were pseudostratified in depth of 1-8 cells in pulpward direction showing the zone of Weil and the cell-rich zone in coronal third pulp. In the central part of pulp tissue, fibroblasts were arranged as a network. These cells strongly immunoreacted with an antibody (monoclonal and polyclonal) directed against the intermediate filament vimentin. The product reaction was specifically located in the cytoplasm. Near vessels occasional lymphocytes and mast cells were also pré¬ sent. Collagen fibers formed a plexus below the cell-rich zone in middle and coronal pulp. KEY WORDS: Pulp - Fibroblast - Man - Histology - Immunohistochemistry. RESUME Vingt dents humaines saines ont été avulsées pour des raisons orthodontiques et traitées pour être exami¬ nées du point de vue histologique et par immunohistochimie. Les odontoblastes étaient pseudostratifiés (1-8 cellules) et dans le tiers coronaire de la pulpe soit la zone de Weil une zone riche en cellules est pré¬ sente. Dans la partie centrale de la pulpe, les fibroblastes forment un réseau. Ces cellules sont fortement immuno-marquées par un anticorps (monoclonal ou polyclonal) dirigé contre la vimentine. Le marquage est spécifiquement localisé dans le cytoplasme. A proximité des vaisseaux, des mastocytes ainsi que des lymphocytes sont présents. -

Effect of Norbixin-Based Poly(Hydroxybutyrate) Membranes on the Tendon Repair Process After Tenotomy in Rats1
ACTA CIRÚRGICA BRASILEIRA ORIGINAL ARTICLE Experimental Surgery Effect of norbixin-based poly(hydroxybutyrate) membranes on the tendon repair process after tenotomy in rats1 Lízia Daniela e Silva NascimentoI , Renata Amadei NicolauII , Antônio Luiz Martins Maia FilhoIII , José Zilton Lima Verde SantosIV , Khetyma Moreira FonsecaV , Danniel Cabral Leão FerreiraVI , Rayssilane Cardoso de SousaVII , Vicente Galber Freitas VianaVIII , Luiz Fernando Meneses CarvalhoIX , José Figueredo-SilvaX I Fellow PhD degree, Postgraduate Program in Biomedical Engineering, Universidade do Vale do Paraíba (UNIVAP), Sao Jose dos Campos-SP. Assistant Professor of Kinesiology, MSc, Health Sciences Center, Physical Therapy, Universidade Estadual do Piauí (UESPI), Teresina-PI, Brazil. Conception, design, intellectual and scientific content of the study; acquisition and interpretation of data; technical procedures; manuscript preparation. II PhD, Collaborator, Postgraduate Program in Biomedical Engineering of the Research and Development Institute, UNIVAP, Sao Jose dos Campos-SP, Brazil. Conception, design, intellectual and scientific content of the study; interpretation of data; critical revision. III Associate Professor of Physiology, Health Sciences Center, UESPI, Teresina-PI, Brazil. Technical procedures. IV MSc, Assistant Professor, Center of Health Sciences, Medicine and Physical Therapy, UESPI, Teresina-PI. Brazil. Histopathological examinations. V Fellow PhD degree, Postgraduate Program in Pharmacology, Universidade Federal do Ceará (UFC), Fortaleza-CE. -

TNF-Α–Stimulated Fibroblasts Secrete Lumican to Promote Fibrocyte Differentiation
TNF-α–stimulated fibroblasts secrete lumican to promote fibrocyte differentiation Darrell Pillinga,1, Varsha Vakilb, Nehemiah Coxa, and Richard H. Gomera,b,1 aDepartment of Biology, Texas A&M University, College Station, TX 77843; and bDepartment of Biochemistry and Cell Biology, Rice University, Houston, TX 77005 Edited by Erica Herzog, Department of Medicine, Yale University, New Haven, CT, and accepted by the Editorial Board August 12, 2015 (received for review April 15, 2015) In healing wounds and fibrotic lesions, fibroblasts and monocyte- lumican levels in the lungs, suggesting that pulmonary fibrosis derived fibroblast-like cells called fibrocytes help to form scar may be in part due to elevated lumican levels. These data suggest tissue. Although fibrocytes promote collagen production by fibro- that lumican may be one of the unknown signals from fibroblasts blasts, little is known about signaling from fibroblasts to fibrocytes. In to fibrocytes that mediates part of a fibroblast-fibrocyte feedback this report, we show that fibroblasts stimulated with the fibrocyte- loop that potentiates fibrosis. secreted inflammatory signal tumor necrosis factor-α secrete the small leucine-rich proteoglycan lumican, and that lumican, but not Results the related proteoglycan decorin, promotes human fibrocyte differ- TNF-α–Stimulated Fibroblasts Promote Fibrocyte Differentiation. To entiation. Lumican competes with the serum fibrocyte differentiation test the hypothesis that activated fibroblasts might secrete soluble inhibitor serum amyloid P, but dominates over the fibroblast-secreted factors that promote fibrocyte differentiation, we added condi- fibrocyte inhibitor Slit2. Lumican acts directly on monocytes, and un- tioned medium from human fibroblasts incubated with TNF-α like other factors that affect fibrocyte differentiation, lumican has no (FCM+TNF-α) to human peripheral blood mononuclear cells detectable effect on macrophage differentiation or polarization. -

On the Reticular Tissue and Lattice=Fibers Occurring in the Milk=Spots of Omentum
On the Reticular Tissue and Lattice=fibers occurring in the Milk=spots of Omentum. By Dr. Yukio Hamazaki. From the Pathological Department of Okayama Medical College (Director: Prof. Oto Tam ura). 2 Figures (Plate III) and 3 Text Figures. Ranvier and Weide n reic h regarded the omentum as a flattened- out lymph gland and the abdominal cavity as its lymph sinus. The latter, furthermore, theoretically emphasized that the omentum is nothing but a sheet of reticular tissue, the "taches laiteuses" correspond- ing to the secondary nodules. Lately, Kiy ono agreed with the above view, though lie pointed out that the histiocytic cells in the milk-spots do not form reticular tissue, unlike those in the lymph glands. At the Fifteenth Pathological Congress of Japan (1925) I reported that the milk-spots in the rat, cattle, and pig are provided with a certain kind of reticular tissue. The purpose of the present paper is to settle this problem using specific stainings for the reticular fibers and for the lattice-fibers ("Gitterfasern" of v. Kupffer), which may be in an intiniate relation with them. Material and methods. The material was obtained from the cattle, pig , dog, cat, rabbit, guinea-pig, rat, mouse, chicken and human subject. As the control the organs containing reticular tissue, i. e., lymph glands , spleen and thymus gland were also examined. The material fixed with 10% solution of lormalin was studied as stretched specimens and as sections . For reticulum-staining the eosin-methyl blue method modified by the author was used: 1. Sections are stained for 30 minutes in 1% solution of eosin (a few drops of glacial acetic acid is added to 100 cc of the solution) . -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -
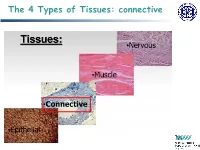
The 4 Types of Tissues: Connective
The 4 Types of Tissues: connective Connective Tissue General structure of CT cells are dispersed in a matrix matrix = a large amount of extracellular material produced by the CT cells and plays a major role in the functioning matrix component = ground substance often crisscrossed by protein fibers ground substance usually fluid, but it can also be mineralized and solid (bones) CTs = vast variety of forms, but typically 3 characteristic components: cells, large amounts of amorphous ground substance, and protein fibers. Connective Tissue GROUND SUBSTANCE In connective tissue, the ground substance is an amorphous gel-like substance surrounding the cells. In a tissue, cells are surrounded and supported by an extracellular matrix. Ground substance traditionally does not include fibers (collagen and elastic fibers), but does include all the other components of the extracellular matrix . The components of the ground substance vary depending on the tissue. Ground substance is primarily composed of water, glycosaminoglycans (most notably hyaluronan ), proteoglycans, and glycoproteins. Usually it is not visible on slides, because it is lost during the preparation process. Connective Tissue Functions of Connective Tissues Support and connect other tissues Protection (fibrous capsules and bones that protect delicate organs and, of course, the skeletal system). Transport of fluid, nutrients, waste, and chemical messengers is ensured by specialized fluid connective tissues, such as blood and lymph. Adipose cells store surplus energy in the form of fat and contribute to the thermal insulation of the body. Embryonic Connective Tissue All connective tissues derive from the mesodermal layer of the embryo . The first connective tissue to develop in the embryo is mesenchyme , the stem cell line from which all connective tissues are later derived. -

Connective Tissues (C.T.)
Lecture 3: Connective tissues (C.T.) - Colours index : Red : important Grey : doctors notes Pink : Girls slides Objectives : 1. Enumerate the general characteristics of C.T. 2. Classify C.T. Into C.T. Proper (C.T.P.) and special types of C.T. 3. Describe components of C.T.P. 4. Classify C.T.P. and know the distribution and function of each type Definition and components of C.T. 1.It is one of the 4 basic tissues. 2.it is Mesodermal* in origin. Function of C.T 1. Supports, binds and connects other tissue and organs. 2. Provides structural (fix organ position) and metabolic support. General characteristics of C.T : 1. It is formed of widely separated, few cells with abundant extracellular matrix. 2. Most of C.T. Are vascular (have blood vessel). Components of C.T : 1. Cells: different types. 2. Fibers: collagenous, elastic & reticular. 3. Matrix: the intercellular substance = extracellular matrix, where cells and fibers are embedded. *Mesodermal: (the middle layer of an embryo in early development, between endoderm and ectoderm) “Referring to embryology” ;) Types of C.T. (Depending on matrix) - Soft = C.T. Proper - Rigid (firm,rubbery) = Cartilage - Hard (solid) = Bone - Fluid = Blood Components of C.T. Proper ● Cells ● Fibers ● Matrix Cells: 1. Fibroblasts 2. Macrophages 3. Mast cells 4. Plasma cells 5. Adipose cells 6. Leucocytes (اﻟﺧﻼﯾﺎ اﻟﻣﻛوﻧﺔ ﻟﻠـCells: (connective tissue ❖ Fibroblast Macrophages Mast Cells ● It’s the most common cell, L/M: L/M: found nearly in all types of C.T ● Basophilic cytoplasm, rich in Cytoplasm contains numerous proper. lysosomes. basophilic and cytoplasmic granules. -

Characterizing the Invasive Tumor Front of Aggressive Uterine Adenocarcinoma and Leiomyosarcoma
Characterizing the invasive tumor front of aggressive uterine adenocarcinoma and leiomyosarcoma Sabina Sanegre1, Núria Eritja2, 3, 4, 5, 6, Carlos de Andrea2, 7, Juan Díaz-Martín2, 8, 9, 10, 11, Ángel Díaz-Lagares2, 12, 13, María Amalia Jácome14, Carmen Salguero-Aranda8, 9, 10, 11, David García-Ros7, Ben Davidson15, 16, 17, Rafael López2, 13, 12, Ignacio Melero2, 18, Samuel Navarro2, 1, Santiago Ramon Y Cajal2, 19, Enrique De Álava2, 8, 9, 10, 11, Xavier Matias-Guiu2, 3, 5, 6, 4*, Rosa Noguera2, 20, 1* 1Institute of Health Research (INCLIVA), Spain, 2Centro de Investigaciónació Biomédica en Red del Cáncer (CIBERONC), Spain, 3Department of Pathology, University Hospital ArnauAr de Vilanova, Spain, 4Bellvitge University Hospital, Spain, 5Universitatt de Lleida, Spain, 6Universityniversity of Barcelona, Spain, 7University Clinic of Navarra, Spain, 8Institutetute of Biomedicine of Seville (IBIS), Spain,Sp 9Virgen del Rocío University Hospital, Spain, 10Consejo Superior de Investigaciones CientífiCientíficas (CSIC), Spain, 11Sevilla University, 12 13 Spain, University Clinical Hospital of Santiago, Spain,Spa Health Research Institute of Santiago de Compostelapostela (IDIS), Spain, 14Facultyculty of Science,Scie University of A Coruña, Spain, 15Institute of Clinical Medicine,ne, Faculty of Medicine,Me University of Oslo, Norway, 16Department of Pathology, Oslo University Hospital,l, Norway,Nor 17Norwegian Radium Hospital, Oslo University Hospital, Norway, 18Departamento de Dermatología,D Clínica Universidad de Navarra, Spain, 19Department of -

Tendon Healing with Allogenic Fibroblast and Static Magnetic Field in Rabbit Model
IJVS 2015; 10(2); Serial No:23 IRANIAN JOURNAL OF VETERINARY SURGERY (IJVS) WWW.IVSA.IR Tendon Healing with Allogenic Fibroblast and Static Magnetic Field in Rabbit Model Amin Bigham-Sadegh*1, Setare Ghasemi 2, Iraj Karimi 3, Pezhman Mirshokraei 4, Hasan Nazari 5, Ahmad Oryan 6 Abstract Objectives- Tendons are integral parts of musculoskeletal system and are subjected to injury. Fibroblast is used in tendon healing, however, there is no proved and reported result regarding concurrent use of allogenic fibroblast with static magnetic field in tendon healing. In addition, there are some studies done on the effect of magnetic fields on tendon healing but the results are antithesis. The aim of this study is to evaluate the effect of simultaneous application of fibroblast and magnetic field on tendon healing in rabbit model. Design- Experimental study. Animals- Eighteen female rabbits, 15 months old and weighing 3.0±0.5 kg were used in this study. Procedures- Two legs of eighteen rabbits were divided into 6 groups. After skin incision, superficial flexor tendon was exposed and cut transversely and then sutured. In control group tendon injury were created in right and left legs and sutured in bunnell mayer suturing technique. In culture media substance group after tendon injury in two legs, 0.5 cc culture substance was injected in the injured tendon area in two legs. In fibroblast group, fibroblast cells were injected in the tendon injured area in both legs. Then all injuries legs were dressed up, a piece of magnet was placed in the surrounding bandage of the left leg for 7 days and right legs were left empty. -

Review: Epithelial Tissue
Review: Epithelial Tissue • “There are 2 basic kinds of epithelial tissues.” What could that mean? * simple vs. stratified * absorptive vs. protective * glands vs. other • You are looking at epithelial cells from the intestine. What do you expect to see? tight junctions; simple columnar; gobet cells; microvilli • You are looking at epithelial cells from the trachea. What do you expect to see? cilia; pseudostratified columnar; goblet cells 1 4-1 Four Types of Tissue Tissue Type Role(s) - Covers surfaces/passages - Forms glands - Structural support CONNECTIVE - Fills internal spaces - Transports materials - Contraction! - Transmits information (electrically) 2 Classification of connective tissue 1. Connective tissue proper 1a. Loose: areolar, adipose, reticular 1b. Dense: dense regular, dense irregular, elastic 2. Fluid connective tissue 2a. Blood: red blood cells, white blood cells, platelets 2b. Lymph 3. Supporting connective tissue 3a. Cartilage: hyaline, elastic, fibrocartilage 3b. Bone 3 Defining connective tissue by the process of elimination if not epithelial, muscle, or nervous, must be connective! 4 LAB MANUAL Figure 6.4 Areolar connective tissue: A prototype (model) connective tissue. Cell types Extracellular matrix Ground substance Macrophage Fibers = proteins • Collagen fiber • Elastic fiber • Reticular fiber Fibroblast Lymphocyte Adipocyte Capillary Mast cell 5 The Cells of Connective Tissue Proper Melanocytes and macrophages, mesenchymal, mast; Adipo- / lympho- / fibrocytes and also fibroblasts. These are the cells of connective -

Índice De Denominacións Españolas
VOCABULARIO Índice de denominacións españolas 255 VOCABULARIO 256 VOCABULARIO agente tensioactivo pulmonar, 2441 A agranulocito, 32 abaxial, 3 agujero aórtico, 1317 abertura pupilar, 6 agujero de la vena cava, 1178 abierto de atrás, 4 agujero dental inferior, 1179 abierto de delante, 5 agujero magno, 1182 ablación, 1717 agujero mandibular, 1179 abomaso, 7 agujero mentoniano, 1180 acetábulo, 10 agujero obturado, 1181 ácido biliar, 11 agujero occipital, 1182 ácido desoxirribonucleico, 12 agujero oval, 1183 ácido desoxirribonucleico agujero sacro, 1184 nucleosómico, 28 agujero vertebral, 1185 ácido nucleico, 13 aire, 1560 ácido ribonucleico, 14 ala, 1 ácido ribonucleico mensajero, 167 ala de la nariz, 2 ácido ribonucleico ribosómico, 168 alantoamnios, 33 acino hepático, 15 alantoides, 34 acorne, 16 albardado, 35 acostarse, 850 albugínea, 2574 acromático, 17 aldosterona, 36 acromatina, 18 almohadilla, 38 acromion, 19 almohadilla carpiana, 39 acrosoma, 20 almohadilla córnea, 40 ACTH, 1335 almohadilla dental, 41 actina, 21 almohadilla dentaria, 41 actina F, 22 almohadilla digital, 42 actina G, 23 almohadilla metacarpiana, 43 actitud, 24 almohadilla metatarsiana, 44 acueducto cerebral, 25 almohadilla tarsiana, 45 acueducto de Silvio, 25 alocórtex, 46 acueducto mesencefálico, 25 alto de cola, 2260 adamantoblasto, 59 altura a la punta de la espalda, 56 adenohipófisis, 26 altura anterior de la espalda, 56 ADH, 1336 altura del esternón, 47 adipocito, 27 altura del pecho, 48 ADN, 12 altura del tórax, 48 ADN nucleosómico, 28 alunarado, 49 ADNn, 28