The Selection and Use of Essential Medicines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amidotrizoic Acid/Barium Sulfate 1477 Imbalance Should Be Corrected Before Contrast Media Are Given
Amidotrizoic Acid/Barium Sulfate 1477 imbalance should be corrected before contrast media are given. management of adhesive small bowel obstruction;2 they allow pyloric stenosis or lesions that may predispose to obstruction. Particular care is needed in patients with multiple myeloma since identification of patients who require surgery and, although they Adequate hydration should be ensured after the procedure to pre- dehydration resulting from use of contrast media may cause pre- have not been shown to relieve obstruction, they may reduce vent severe constipation. cipitation of protein in the renal tubules, leading to anuria and length of hospital stay in patients treated without surgery. It is contra-indicated in patients with gastrointestinal perforation, fatal renal failure. 1. Murshed R, et al. Meconium ileus: a ten-year review of thirty-six and should be avoided, particularly when given rectally, in those Caution is also necessary in patients with severe hypertension, patients. Eur J Pediatr Surg 1997; 7: 275–7. at risk of perforation, such as patients with acute ulcerative colitis advanced cardiac disease, phaeochromocytoma, sickle-cell dis- 2. Abbas S, et al. Oral water soluble contrast for the management or diverticulitis and after rectal or colonic biopsy, sigmoidosco- of adhesive small bowel obstruction. Available in The Cochrane ease, or hyperthyroidism or epilepsy, and in debilitated, severely Database of Systematic Reviews; Issue 3. Chichester: John Wi- py, or radiotherapy. ill, very old, or very young patients. ley; 2007 (accessed 14/07/08). Uses and Administration Amidotrizoates and other hypertonic contrast media are neuro- Preparations Barium sulfate is used as a radiographic contrast medium toxic and should not be given intrathecally; patients with sub- (p.1474) for X-ray examination of the gastrointestinal tract in- arachnoid haemorrhage may be at risk with any intravascular BP 2008: Meglumine Amidotrizoate Injection; Sodium Amidotrizoate In- jection; volving single- or double-contrast techniques or computed tom- use. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -
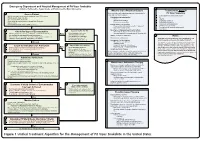
Snake Bite Protocol
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

The Impact of a Changed Legislation on Reporting of Adverse Drug Reactions in Sweden, with Focus on Nurses Reporting
The impact of a changed legislation on reporting of adverse drug reactions in Sweden, with focus on nurses reporting Sofia A. Karlsson, Ingela Jacobsson, Marit Danell Boman, Katja M. Hakkarainen, Henrik Lövborg, Staffan Hägg and Anna K Jönsson Linköping University Post Print N.B.: When citing this work, cite the original article. The original publication is available at www.springerlink.com: Sofia A. Karlsson, Ingela Jacobsson, Marit Danell Boman, Katja M. Hakkarainen, Henrik Lövborg, Staffan Hägg and Anna K Jönsson, The impact of a changed legislation on reporting of adverse drug reactions in Sweden, with focus on nurses reporting, 2015, European Journal of Clinical Pharmacology, (71), 5, 631-636. http://dx.doi.org/10.1007/s00228-015-1839-6 Copyright: Springer Verlag (Germany) http://www.springerlink.com/?MUD=MP Postprint available at: Linköping University Electronic Press http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-118037 The impact of a changed legislation on reporting of adverse drug reactions in Sweden, with focus on nurses’ reporting Sofia A Karlsson1, Ingela Jacobsson2, Marit Danell Boman3, Katja M Hakkarainen4,5, Henrik Lövborg2, Staffan Hägg2,5, Anna K Jönsson2 Affiliations: 1. Department of Public Health and Community Medicine, the Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden 2. Department of Clinical Pharmacology and Department of Medical and Health Sciences, Linköping University, Linköping, Sweden 3. Division of Clinical Pharmacology, University Hospital of Umeå, Umeå, Sweden 4. Nordic School of -

Assessing the Availability, Service Quality, and Price of Essential Medicines In
Assessing the Availability, Service Quality, and Price of Essential Medicines in Private Pharmacies in Afghanistan Norio Kasahara A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2015 Reading Committee: Louis P. Garrison, Jr., Chair Joseph B. Babigumira Andy Stergachis Program Authorized to Offer Degree: Pharmaceutical Outcomes Research and Policy ©Copyright 2015 Norio Kasahara ii Table of Contents Abstract ................................................................................................................................................................................... ................................................................................................ ................................................................................................ .................................................................................. ............... vvv Acknowledgements ................................................................................................................................................................................... ................................................................................................ ................................................................................. ............ viiviivii Summary ................................................................................................................................................................................... ............................................................................................... -

Reseptregisteret 2013–2017 the Norwegian Prescription Database
LEGEMIDDELSTATISTIKK 2018:2 Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Christian Berg Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Utgitt av Folkehelseinstituttet/Published by Norwegian Institute of Public Health Område for Helsedata og digitalisering Avdeling for Legemiddelstatistikk Juni 2018 Tittel/Title: Legemiddelstatistikk 2018:2 Reseptregisteret 2013–2017 / The Norwegian Prescription Database 2013–2017 Forfattere/Authors: Christian Berg, redaktør/editor Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Acknowledgement: Julie D. W. Johansen (English text) Bestilling/Order: Rapporten kan lastes ned som pdf på Folkehelseinstituttets nettsider: www.fhi.no The report can be downloaded from www.fhi.no Grafisk design omslag: Fete Typer Ombrekking: Houston911 Kontaktinformasjon/Contact information: Folkehelseinstituttet/Norwegian Institute of Public Health Postboks 222 Skøyen N-0213 Oslo Tel: +47 21 07 70 00 ISSN: 1890-9647 ISBN: 978-82-8082-926-9 Sitering/Citation: Berg, C (red), Reseptregisteret 2013–2017 [The Norwegian Prescription Database 2013–2017] Legemiddelstatistikk 2018:2, Oslo, Norge: Folkehelseinstituttet, 2018. Tidligere utgaver / Previous editions: 2008: Reseptregisteret 2004–2007 / The Norwegian Prescription Database 2004–2007 2009: Legemiddelstatistikk 2009:2: Reseptregisteret 2004–2008 / The Norwegian Prescription Database 2004–2008 2010: Legemiddelstatistikk 2010:2: Reseptregisteret 2005–2009. Tema: Vanedannende legemidler / The Norwegian Prescription Database 2005–2009. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

Middelinteracties Bij Patiënten Met Kanker, Behandeld Met Orale Antikankermiddelen
OVERZICHTSARTIKELEN 4 Prevalentie van potentiële genees- middelinteracties bij patiënten met kanker, behandeld met orale antikankermiddelen Prevalence of potential drug-drug interactions in cancer patients treated with oral anti-cancer drugs drs. F.M. de Man1*, drs. F. Piran2*, D.H.S. Brundel3, prof. dr. C. Neef4, prof. dr. T. van Gelder5, prof. dr. R.H.J. Mathijssen6, prof. dr. D.M. Burger7, dr. F.G.A. Jansman8 en drs. R.W.F. van Leeuwen9 Samenvatting Achtergrond: Potentiële geneesmiddelinteracties (PGI’s) Resultaten: Bij 898 geïncludeerde patiënten werden bij komen veelvuldig voor bij kankerpatiënten, maar zijn tot 426 patiënten (46%; 95%-betrouwbaarheidsinterval 42- op heden onvoldoende gekwantificeerd bij patiënten 50%) in totaal 1.359 PGI’s gevonden. Bij 143 patiënten met orale antikankerbehandeling. In dit onderzoek wordt (16%) werd een ernstige PGI gevonden. Coumarines de prevalentie en ernst van PGI’s bij poliklinische patiënten en opioïden waren het meest betrokken bij PGI’s. In de behandeld met orale antikankermiddelen beschreven. meerderheid van de gevallen was er sprake van een Methode: In 3 Nederlandse ziekenhuizen zijn via het interactie met betrekking tot het centrale zenuwstelsel, elektronische medicatievoorschrijfsysteem alle patiën- maag-darmtoxiciteit of QTc-verlenging op het elektro- ten met orale antikankermedicatie geselecteerd. PGI’s cardiogram. werden met behulp van elektronische en additionele Conclusie: PGI’s komen veel voor bij kankerpatiënten handmatige screeningsmethoden geïdentificeerd. De met orale antikankertherapie. Artsen en apothekers interacties werden geclassificeerd naar mate van ernst moeten zich bewust zijn van deze mogelijke interacties. en wetenschappelijk bewijs. (Ned Tijdschr Oncol 2014;11:133-40) Summary Background: Potential drug-drug-interactions (PDDIs) Methods: A search was conducted in a computer based in patients with cancer are common, but have not pre- medication prescription system for dispensing oral anti- viously been quantified for oral anti-cancer treatment. -

COMPARISON of the WHO ATC CLASSIFICATION & Ephmra/Intellus Worldwide ANATOMICAL CLASSIFICATION
COMPARISON OF THE WHO ATC CLASSIFICATION & EphMRA/Intellus Worldwide ANATOMICAL CLASSIFICATION: VERSION June 2019 2 Comparison of the WHO ATC Classification and EphMRA / Intellus Worldwide Anatomical Classification The following booklet is designed to improve the understanding of the two classification systems. The development of the two systems had previously taken place separately. EphMRA and WHO are now working together to ensure that there is a convergence of the 2 systems rather than a divergence. In order to better understand the two classification systems, we should pay attention to the way in which substances/products are classified. WHO mainly classifies substances according to the therapeutic or pharmaceutical aspects and in one class only (particular formulations or strengths can be given separate codes, e.g. clonidine in C02A as antihypertensive agent, N02C as anti-migraine product and S01E as ophthalmic product). EphMRA classifies products, mainly according to their indications and use. Therefore, it is possible to find the same compound in several classes, depending on the product, e.g., NAPROXEN tablets can be classified in M1A (antirheumatic), N2B (analgesic) and G2C if indicated for gynaecological conditions only. The purposes of classification are also different: The main purpose of the WHO classification is for international drug utilisation research and for adverse drug reaction monitoring. This classification is recommended by the WHO for use in international drug utilisation research. The EphMRA/Intellus Worldwide classification has a primary objective to satisfy the marketing needs of the pharmaceutical companies. Therefore, a direct comparison is sometimes difficult due to the different nature and purpose of the two systems. -

Long-Term Effects of Snake Envenoming
toxins Review Long-Term Effects of Snake Envenoming Subodha Waiddyanatha 1,2, Anjana Silva 1,2 , Sisira Siribaddana 1 and Geoffrey K. Isbister 2,3,* 1 Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka, Saliyapura 50008, Sri Lanka; [email protected] (S.W.); [email protected] (A.S.); [email protected] (S.S.) 2 South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, University of Peradeniya, Peradeniya 20400, Sri Lanka 3 Clinical Toxicology Research Group, University of Newcastle, Callaghan, NSW 2308, Australia * Correspondence: [email protected] or [email protected]; Tel.: +612-4921-1211 Received: 14 March 2019; Accepted: 29 March 2019; Published: 31 March 2019 Abstract: Long-term effects of envenoming compromise the quality of life of the survivors of snakebite. We searched MEDLINE (from 1946) and EMBASE (from 1947) until October 2018 for clinical literature on the long-term effects of snake envenoming using different combinations of search terms. We classified conditions that last or appear more than six weeks following envenoming as long term or delayed effects of envenoming. Of 257 records identified, 51 articles describe the long-term effects of snake envenoming and were reviewed. Disability due to amputations, deformities, contracture formation, and chronic ulceration, rarely with malignant change, have resulted from local necrosis due to bites mainly from African and Asian cobras, and Central and South American Pit-vipers. Progression of acute kidney injury into chronic renal failure in Russell’s viper bites has been reported in several studies from India and Sri Lanka. Neuromuscular toxicity does not appear to result in long-term effects. -

1 EMA Tender EMA/2017/09/PE, Lot 2 Impact of EU Label
EMA tender EMA/2017/09/PE, Lot 2 Impact of EU label changes and revised pregnancy prevention programme for oral retinoid containing medicinal products: risk awareness and adherence Protocol • Prof. Anna Birna Almarsdóttir, Professor in Social and Clinical Pharmacy at the Department of Pharmacy, Faculty of Health and Medical Sciences, University of Copenhagen • Prof. Marcel Bouvy, Professor of Pharmaceutical Care at the Division of Pharmacoepidemiology & Clinical Pharmacology of the Department of Pharmaceutical Sciences, Utrecht University. • Dr Rob Heerdink, Associate Professor at the Division of Pharmacoepidemiology & Clinical Pharmacology of the Department of Pharmaceutical Sciences, Utrecht University. • Dr Teresa Leonardo Alves, Researcher at the Centre for Health Protection, National Institute for Public Health and the Environment, The Netherlands. 1 Table of contents Background ...................................................................................................................... 3 Aims of the study ............................................................................................................. 4 Methods ........................................................................................................................... 4 Setting ........................................................................................... Error! Bookmark not defined. Study design ............................................................................................................................ 4 Population