Radionuclide Investigations of the Urinary Tract in the Era of Multimodality Imaging*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urology Services in the ASC
Urology Services in the ASC Brad D. Lerner, MD, FACS, CASC Medical Director Summit ASC President of Chesapeake Urology Associates Chief of Urology Union Memorial Hospital Urologic Consultant NFL Baltimore Ravens Learning Objectives: Describe the numerous basic and advanced urology cases/lines of service that can be provided in an ASC setting Discuss various opportunities regarding clinical, operational and financial aspects of urology lines of service in an ASC setting Why Offer Urology Services in Your ASC? Majority of urologic surgical services are already outpatient Many urologic procedures are high volume, short duration and low cost Increasing emphasis on movement of site of service for surgical cases from hospitals and insurance carriers to ASCs There are still some case types where patients are traditionally admitted or placed in extended recovery status that can be converted to strictly outpatient status and would be suitable for an ASC Potential core of fee-for-service case types (microsurgery, aesthetics, prosthetics, etc.) Increasing Population of Those Aged 65 and Over As of 2018, it was estimated that there were 51 million persons aged 65 and over (15.63% of total population) By 2030, it is expected that there will be 72.1 million persons aged 65 and over National ASC Statistics - 2017 Urology cases represented 6% of total case mix for ASCs Urology cases were 4th in median net revenue per case (approximately $2,400) – behind Orthopedics, ENT and Podiatry Urology comprised 3% of single specialty ASCs (5th behind -

Native Kidney Biopsy
Mohammed E, et al., J Nephrol Renal Ther 2020, 6: 034 DOI: 10.24966/NRT-7313/100034 HSOA Journal of Nephrology & Renal Therapy Review Article Native Kidney Biopsy: An Introduction The burden of non communicable diseases has been a worldwide Update and Best Practice public health challenge, as chronic diseases compose 61% of global deaths and 49% of the global burden of diseases. Currently, many Evidence countries are encountering a fast transformation in the disease pro- file from first generation diseases such as infectious diseases to the encumbrance of non communicable diseases. In addition, Chronic Ehab Mohammed1, Issa Al Salmi1 *, Shilpa Ramaiah1 and Suad Hannawi2 Kidney Disease (CKD) is increasingly recognized as a global public health challenge as 10% of the global population is affected [1,2]. 1Nephrologist, The Renal Medicine Department, The Royal Hospital, Muscat, Oman The scarcity of well-trained renal pathologists, even in high-in- come countries, is a major obstacle to use of biopsy samples. The ISN 2Medicine Department, Ministry of Health and Prevention, Dubai, UAE is working worldwide to enhance development of local renal patholo- gy expertise. Levin et al stated that analysis of kidney biopsy samples can be used to stratify CKD into distinct subgroups of diseases based Abstract on specific histological patterns, when combined with the clinical pre- sentation [3]. Diabetes mellitus and hypertensive nephropathy are the Objectives: To Provide up-to-date guidelines for medical and nurs- commonly identified causes of End-Stage Kidney Disease (ESKD). ing staffs on the pre, during, and post care of a patient undergoing a Also, many patients with glomerulonephritis, systemic lupus erythe- percutaneous-kidney-biopsy-PKB. -

Nuclear Medicine for Medical Students and Junior Doctors
NUCLEAR MEDICINE FOR MEDICAL STUDENTS AND JUNIOR DOCTORS Dr JOHN W FRANK M.Sc, FRCP, FRCR, FBIR PAST PRESIDENT, BRITISH NUCLEAR MEDICINE SOCIETY DEPARTMENT OF NUCLEAR MEDICINE, 1ST MEDICAL FACULTY, CHARLES UNIVERSITY, PRAGUE 2009 [1] ACKNOWLEDGEMENTS I would very much like to thank Prof Martin Šámal, Head of Department, for proposing this project, and the following colleagues for generously providing images and illustrations. Dr Sally Barrington, Dept of Nuclear Medicine, St Thomas’s Hospital, London Professor Otakar Bělohlávek, PET Centre, Na Homolce Hospital, Prague Dr Gary Cook, Dept of Nuclear Medicine, Royal Marsden Hospital, London Professor Greg Daniel, formerly at Dept of Veterinary Medicine, University of Tennessee, currently at Virginia Polytechnic Institute and State University (Virginia Tech), Past President, American College of Veterinary Radiology Dr Andrew Hilson, Dept of Nuclear Medicine, Royal Free Hospital, London, Past President, British Nuclear Medicine Society Dr Iva Kantorová, PET Centre, Na Homolce Hospital, Prague Dr Paul Kemp, Dept of Nuclear Medicine, Southampton University Hospital Dr Jozef Kubinyi, Institute of Nuclear Medicine, 1st Medical Faculty, Charles University Dr Tom Nunan, Dept of Nuclear Medicine, St Thomas’s Hospital, London Dr Kathelijne Peremans, Dept of Veterinary Medicine, University of Ghent Dr Teresa Szyszko, Dept of Nuclear Medicine, St Thomas’s Hospital, London Ms Wendy Wallis, Dept of Nuclear Medicine, Charing Cross Hospital, London Copyright notice The complete text and illustrations are copyright to the author, and this will be strictly enforced. Students, both undergraduate and postgraduate, may print one copy only for personal use. Any quotations from the text must be fully acknowledged. It is forbidden to incorporate any of the illustrations or diagrams into any other work, whether printed, electronic or for oral presentation. -

Nuclear Pharmacy Quick Sample
12614-01_CH01-rev3.qxd 10/25/11 10:56 AM Page 1 CHAPTER 1 Radioisotopes Distribution for Not 1 12614-01_CH01-rev3.qxd 10/25/1110:56AMPage2 2 N TABLE 1-1 Radiopharmaceuticals Used in Nuclear Medicine UCLEAR Chemical Form and Typical Dosage P Distribution a b HARMACY Radionuclide Dosage Form Use (Adult ) Route Carbon C 11 Carbon monoxide Cardiac: Blood volume measurement 60–100 mCi Inhalation Carbon C 11 Flumazenil injection Brain: Benzodiazepine receptor imaging 20–30 mCi IV Q UICK Carbon C 11 Methionine injection Neoplastic disease evaluation in brain 10–20 mCi IV R Carbon C 11 forRaclopride injection Brain: Dopamine D2 receptor imaging 10–15 mCi IV EFERENCE Carbon C 11 Sodium acetate injection Cardiac: Marker of oxidative metabolism 12–40 mCi IV Carbon C 14 Urea Diagnosis of Helicobacter pylori infection 1 µCi PO Chromium Cr 51 Sodium chromate injection Labeling red blood cells (RBCs) for mea- 10–80 µCi IV suring RBC volume, survival, and splenic sequestration Cobalt Co 57 Cyanocobalamin capsules Diagnosis of pernicious anemia and 0.5 µCi PO Not defects of intestinal absorption Fluorine F 18 Fludeoxyglucose injection Glucose utilization in brain, cardiac, and 10–15 mCi IV neoplastic disease Fluorine F 18 Fluorodopa injection Dopamine neuronal decarboxylase activity 4–6 mCi IV in brain Fluorine F 18 Sodium fluoride injection Bone imaging 10 mCi IV Gallium Ga 67 Gallium citrate injection Hodgkin’s disease, lymphoma 8–10 mCi IV Acute inflammatory lesions 5 mCi IV Indium In 111 Capromab pendetide Metastatic imaging in patients with biopsy- -

Percutaneous Ultrasound-Guided Renal Biopsy; a Comparison of Axial Vs
Percutaneous ultrasound-guided renal biopsy; A comparison of axial vs. sagittal probe location FARNAZ SHAMSHIRGAR1, SEYED MORTEZA BAGHERI2* 1Resident of Radiology, Iran University of Medical Sciences, Tehran, Iran 2Department of Radiology, Hasheminejad Kidney Center (HKC), Iran University of Medical Sciences, Tehran, Iran Background. Renal biopsy is an important method for diagnosis of renal parenchymal abnormalities. Here, we compare the effectiveness and complications of percutaneous ultrasound- guided renal biopsy using axial vs. sagittal probe locations. Methods. In a cross-sectional survey, in 2012, patients with a nephrologist order were biopsied by a radiology resident. Renal biopsy was done on 15 patients using axial (A group) and the same number of biopsies done with sagittal probe location (S group). The two groups were compared in term of the yields and complications of each method. Results. In the A group, the ratio of glomeruli gathered to the number of obtained samples was significantly higher than in the S group. Nine patients in the A group (60%) required only two samplings, whereas 66.7% in the S group required more than two attempts. Microscopic hematuria was more common in the A; conversely, gross hematuria was less common in the A group. Meagre hematomas were more frequent in the S group .When compared with hemoglobin level before biopsy, its level 24 hours after biopsy was similar within groups. Conclusion. Our study shows that percutaneous ultrasound-guided renal biopsy using axial probe provides better yield with fewer efforts and fewer serious complications. Keywords: Percutaneous renal biopsy, Ultrasound-guided renal biopsy, Ultra-sonography probe location. INTRODUCTION plications and related post therapeutic side effects may cause another complication such as vascular Percutaneous renal biopsy is an important occlusion, acute obstruction of renal output, renal method to diagnose most kidney diseases. -
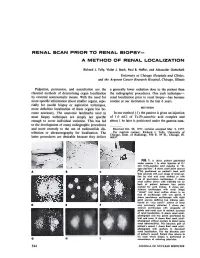
Renal Scan Prior to Renal Biopsy—
RENAL SCAN PRIOR TO RENAL BIOPSY— A METHOD OF RENAL LOCALIZATION Richard J. Tully, Violet J. Stark, Paul B. Hoffer, and Alexander Gottschalk University of Chicago Hospitals and Clinics, and the A rgonne Cancer Research Hospital, Chicago, Illinois Palpation, percussion, and auscultation are the a generally lower radiation dose to the patient than classical methods of determining organ localization the radiographie procedures. One such technique— by external nontraumatic means. With the need for renal localization prior to renal biopsy—has become more specific information about smaller organs, espe routine at our institution in the last 6 years. cially for needle biopsy or aspiration techniques, more definitive localization of these organs has be METHODS come necessary. The anatomic landmarks used in In our method (7) the patient is given an injection most biopsy techniques are simply not specific of 1.0 mCi of Tc-Fe-ascorbic acid complex and enough to cover individual variation. This has led about 1 hr later is positioned under the gamma cam- to the development of many radiographie procedures and more recently to the use of radionuclide dis Received Oct. 28, 1971; revision accepted Mar. 2, 1972. tribution or ultrasonography for localization. The For reprints contact: Richard J. Tully, University of Chicago, Dept. of Radiology, 950 E. 59 St., Chicago, 111. latter procedures are desirable because they deliver 60637. FIG. 1. A shows patient positioned under camera 1 hr after injection of 0.1 mCi Tc-Fe-ascorbic acid complex in "bi opsy position". B shows small point sources ("Co) positioned on patient's back until they coincide with scan image of renal out line by trial and error method or with use of persistence oscilloscope. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Uroradiology Tutorial for Medical Students Lesson 3: Cystography & Urethrography – Part 1
Uroradiology Tutorial For Medical Students Lesson 3: Cystography & Urethrography – Part 1 American Urological Association Introduction • Conventional radiography of the urinary tract includes several diagnostic studies: – Cystogram – Retrograde urethrogram – Voiding cystourethrogram • All of these studies answer questions that are essential to urologic patient management Voiding Cystourethrogram (VCUG) • The voiding cystourethrogram is a dynamic test used to define the anatomy and, in part, the function of the lower urinary tract. It is performed by placing a catheter through the urethra into the bladder, filling the bladder with contrast material and then taking x-rays while the patient voids. You can imagine how popular it is among children. Scout Film • Several films are taken when performing a VCUG. The first image is a KUB called the scout film. On this film one can evaluate the bones of the spine and pelvis (injury or congenital anomaly such as spina bifida) and the soft tissues (calcifications, foreign bodies, etc.). • Normal scout image • What gender? Scout Film • Patients with urologic problems (urine infection or incontinence) may have a spinal abnormality that results in abnormal innervation of the bladder. Such anomalies are commonly associated with anomalies of the vertebral column. Let’s look at some spines. • Here is a spine from a normal KUB or scout film. Notice that the posterior processes of all the vertebrae are intact. You can see the posterior process behind and below each vertebral body. • Here is another scout film. Notice that the posterior processes are absent below L-4. This patient has lower lumbar spina bifida. Read This Scout Film • The bones are normal • What about soft tissues (bowel, etc.)? • This child has significant constipation. -

FDA-Approved Radiopharmaceuticals
Medication Management FDA-approved radiopharmaceuticals This is a current list of all FDA-approved radiopharmaceuticals. USP <825> requires the use of conventionally manufactured drug products (e.g., NDA, ANDA) for Immediate Use. Nuclear medicine practitioners that receive radiopharmaceuticals that originate from sources other than the manufacturers listed in these tables may be using unapproved copies. Radiopharmaceutical Manufacturer Trade names Approved indications in adults (Pediatric use as noted) 1 Carbon-11 choline Various - Indicated for PET imaging of patients with suspected prostate cancer recurrence based upon elevated blood prostate specific antigen (PSA) levels following initial therapy and non-informative bone scintigraphy, computerized tomography (CT) or magnetic resonance imaging (MRI) to help identify potential sites of prostate cancer recurrence for subsequent histologic confirmation 2 Carbon-14 urea Halyard Health PYtest Detection of gastric urease as an aid in the diagnosis of H.pylori infection in the stomach 3 Copper-64 dotatate Curium Detectnet™ Indicated for use with positron emission tomography (PET) for localization of somatostatin receptor positive neuroendocrine tumors (NETs) in adult patients 4 Fluorine-18 florbetaben Life Molecular Neuraceq™ Indicated for Positron Emission Tomography (PET) imaging of the brain to Imaging estimate β amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease (AD) or other causes of cognitive decline 5 Fluorine-18 -

Post-Percutaneous Renal Biopsy Observation Time; Single Center Experience
Open Access Austin Journal of Nephrology and Hypertension Special Article - Chronic Kidney Disease Post-Percutaneous Renal Biopsy Observation Time; Single Center Experience Habas E1*, Elhabash B2, Rayani A3, Turgman F4 and Tarsien R2 Abstract 1 Department of Internal Medicine, Tripoli Central Background: Percutaneous Renal Biopsy (PRB) should be performed Hospital, Libya to diagnose renal damage, to assess response to treatment and to predict 2Department of Rheumatology, Tripoli Medical Center, prognosis.PRB is a safer procedure and mostly free of complications. Assessing Libya optimal time duration post-PRB is important to predict Post-PRB complication 3Department of Pediatrics, Tripoli Children Hospital, and to reduce the cost of PRB. Pediatric Arab Board, Libya 4Pathologist, Tripoli Central Hospital, University of Aim: To reduce the Post-PRB observation time for optimal outcome and Tripoli, Libya patient’s safety. *Corresponding author: Elmukhtar Habas, Methods: All PRBs were performed at the Nephrology Unit, Tripoli Department of Internal Medicine, Tripoli Central Central Hospital, Libya between May 2008 to December 2015. One hundred Hospital, Facharzt Nephrology, Libya eighteen ultrasound-guided PRBs were done. After explaining the procedure and its possible complications, an informed consent was signed by patients. Received: July 01, 2016; Accepted: August 23, 2016; Coagulation profile PT, PTT, INR, BT, CT and CBC were done before PRB. Published: August 25, 2016 Each biopsy was performed with an automated biopsy gun with a 16 -gauge needle under real-time US. Two biopsy specimens from lower pole of left kidney were taken from native kidneys. All patients were kept under close medical supervision and on bed rest for 2-hours. -

Radionuclide Cystography 138
The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College is a nonprofit professional society whose primary purposes are to advance the science of radiology, improve radiologic services to the patient, study the socioeconomic aspects of the practice of radiology, and encourage continuing education for radiologists, radiation oncologists, medical physicists, and persons practicing in allied professional fields. The American College of Radiology will periodically define new practice guidelines and technical standards for radiologic practice to help advance the science of radiology and to improve the quality of service to patients throughout the United States. Existing practice guidelines and technical standards will be reviewed for revision or renewal, as appropriate, on their fifth anniversary or sooner, if indicated. Each practice guideline and technical standard, representing a policy statement by the College, has undergone a thorough consensus process in which it has been subjected to extensive review, requiring the approval of the Commission on Quality and Safety as well as the ACR Board of Chancellors, the ACR Council Steering Committee, and the ACR Council. The practice guidelines and technical standards recognize that the safe and effective use of diagnostic and therapeutic radiology requires specific training, skills, and techniques, as described in each document. Reproduction or modification of the published practice guideline and technical standard by those entities not providing these services is not authorized. Revised 2010 (Res. 25)* ACR–SPR–SNM PRACTICE GUIDELINE FOR THE PERFORMANCE OF ADULT AND PEDIATRIC RADIONUCLIDE CYSTOGRAPHY PREAMBLE These guidelines are an educational tool designed to assist Therefore, it should be recognized that adherence to these practitioners in providing appropriate radiologic care for guidelines will not assure an accurate diagnosis or a patients. -
Procedure Procedure Code Description Rate 500
Procedure Procedure Code Description Rate 500 HEPATOTOMY $0.00 50010 RENAL EXPLORATION, NOT NECESSITATING OTHER SPECIFIC PROCEDURES $433.85 50020 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; OPEN $336.00 50021 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; PERCUTANIOUS $128.79 50040 NEPHROSTOMY, NEPHROTOMY WITH DRAINAGE $420.00 50045 NEPHROTOMY, WITH EXPLORATION $420.00 50060 NEPHROLITHOTOMY; REMOVAL OF CALCULUS $512.40 50065 NEPHROLITHOTOMY; SECONDARY SURGICAL OPERATION FOR CALCULUS $512.40 50070 NEPHROLITHOTOMY; COMPLICATED BY CONGENITAL KIDNEY ABNORMALITY $512.40 NEPHROLITHOTOMY; REMOVAL OF LARGE STAGHORN CALCULUS FILLING RENAL 50075 PELVIS AND CALYCES (INCLUDING ANATROPHIC PYE $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50080 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50081 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 501 DIAGNOSTIC PROCEDURES ON LIVER $0.00 TRANSECTION OR REPOSITIONING OF ABERRANT RENAL VESSELS (SEPARATE 50100 PROCEDURE) $336.00 5011 CLOSED (PERCUTANEOUS) (NEEDLE) BIOPSY OF LIVER $0.00 5012 OPEN BIOPSY OF LIVER $0.00 50120 PYELOTOMY; WITH EXPLORATION $420.00 50125 PYELOTOMY; WITH DRAINAGE, PYELOSTOMY $420.00 5013 TRANSJUGULAR LIVER BIOPSY $0.00 PYELOTOMY; WITH REMOVAL OF CALCULUS (PYELOLITHOTOMY, 50130 PELVIOLITHOTOMY, INCLUDING COAGULUM PYELOLITHOTOMY) $504.00 PYELOTOMY; COMPLICATED (EG, SECONDARY OPERATION, CONGENITAL KIDNEY 50135 ABNORMALITY) $504.00 5014 LAPAROSCOPIC LIVER BIOPSY $0.00 5019 OTHER DIAGNOSTIC PROCEDURES