Thallium from Wikipedia, the Free Encyclopedia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Thallium-201 for Medical Use. I
THALLIUM-201 FOR MEDICAL USE. I E. Lebowitz, M. W. Greene, R. Fairchild, P. R. Bradley-Moore, H. 1. Atkins,A. N. Ansari, P. Richards,and E. Belgrave Brookhaven National Laboratory Thallium-201 merits evaluation for myocar tumors (7—9), the use of radiothallium should also dial visualization, kidney studies, and tumor be evaluated for this application. diagnosis because of its physical and biologic Thallium-201 decays by electron capture with a properties. A method is described for prepara 73-hr half-life. It emits mercury K-x-rays of 69—83 tion of this radiopharmaceutical for human use. keY in 98% abundance plus gamma rays of I 35 and A critical evaluation of 501T1 and other radio 167 keV in 10% total abundance. Because of its pharmaceuticals for myocardial visualization is good shelf-life, photon energies, and mode of decay, given. 201T1was the radioisotope of thallium chosen for development. Thallium-20 1 is a potentially useful radioisotope MATERIALS AND METHODS for various medical applications including myocardial Thallium-201 is produced by irradiating a natural visualization and possible assessment of physiology, thallium target in the external beam of the 60-in. as a renal medullary imaging agent, and for tumor Brookhaven cyclotron with 3 1-MeV protons. The detection. nuclear reaction is 203Tl(p,3n)201Pb. Lead-201 has The use of radiothallium in nuclear medicine was a half-life of 9.4 hr and is the parent of 201T1.The first suggested by Kawana, et al (1 ) . In terms of thallium target, fabricated from an ingot of 99.999% organ distribution (2) and neurophysiologic function pure natural thallium metal (29.5% isotopic abun (3), thallium is biologically similar to potassium. -

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

Effect of Natural Organic Matter on Thallium and Silver Speciation Loïc Martin, Caroline Simonucci, Sétareh Rad, Marc F
Effect of natural organic matter on thallium and silver speciation Loïc Martin, Caroline Simonucci, Sétareh Rad, Marc F. Benedetti To cite this version: Loïc Martin, Caroline Simonucci, Sétareh Rad, Marc F. Benedetti. Effect of natural organic matter on thallium and silver speciation. Journal of Environmental Sciences, Elsevier, 2020, 93, pp.185-192. 10.1016/j.jes.2020.04.001. hal-02565435 HAL Id: hal-02565435 https://hal.archives-ouvertes.fr/hal-02565435 Submitted on 6 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Title Page (with Author Details) Effect of natural organic matter on thallium and silver speciation Loïc A. Martin1,2, Caroline Simonucci2, Sétareh Rad3, and Marc F. Benedetti1* 1Université de Paris, Institut de physique du Globe de Paris, CNRS, F-75005 Paris, France. 2IRSN, PSE-ENV/SIRSE/LER-Nord, BP 17, 92262 Fontenay-aux-Roses Cedex, France 3BRGM, Unité de Géomicrobiologie et Monitoring environnemental 45060 Orléans Cedex 2, France. *Corresponding authors. Email address: [email protected] Manuscript File Click here to access/download;Graphical Abstract;graphical abstract.001.jpeg Manuscript File Click here to view linked References 23 Effect of natural organic matter on thallium and silver speciation 24 25 Loïc A. -

Rare Earth Elements: the Global Supply Chain
Rare Earth Elements: The Global Supply Chain Marc Humphries Analyst in Energy Policy September 30, 2010 Congressional Research Service 7-5700 www.crs.gov R41347 CRS Report for Congress Prepared for Members and Committees of Congress Rare Earth Elements: The Global Supply Chain Summary The concentration of production of rare earth elements (REEs) outside the United States raises the important issue of supply vulnerability. REEs are used for new energy technologies and national security applications. Is the United States vulnerable to supply disruptions of REEs? Are these elements essential to U.S. national security and economic well-being? There are 17 rare earth elements (REEs), 15 within the chemical group called lanthanides, plus yttrium and scandium. The lanthanides consist of the following: lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium. Rare earths are moderately abundant in the earth’s crust, some even more abundant than copper, lead, gold, and platinum. While more abundant than many other minerals, REE are not concentrated enough to make them easily exploitable economically. The United States was once self-reliant in domestically produced REEs, but over the past 15 years has become 100% reliant on imports, primarily from China, because of lower-cost operations. There is no rare earth mine production in the United States. U.S.-based Molycorp operates a separation plant at Mountain Pass, CA, and sells the rare earth concentrates and refined products from previously mined above-ground stocks. Neodymium, praseodymium, and lanthanum oxides are produced for further processing but these materials are not turned into rare earth metal in the United States. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -
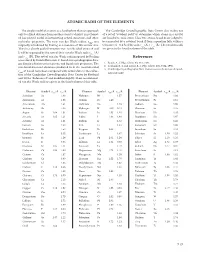
ATOMIC RADII of the ELEMENTS References
ATOMIC RADII OF THE ElEMENTS The simple model of an atom as a hard sphere that can approach The Cambridge Crystallographic Data Center also makes use only to a fixed distance from another atom to which it is not bond- of a set of “covalent radii” to determine which atoms in a crystal ed has proved useful in interpreting crystal structures and other are bonded to each other . Thus two atoms A and B are judged to molecular properties . The term van der Waals radius, rvdw, was be connected by a covalent bond if their separation falls within a originally introduced by Pauling as a measure of this atomic size . tolerance of ±0 .4 Å of the sum rcov (A) + rcov (B) . The covalent radii Thus in a closely packed structure two non-bonded atoms A and are given in the fourth column of the table . B will be separated by the sum of their van der Waals radii rvdw (A) and rvdw (B) . The set of van der Waals radii proposed by Pauling References was refined by Bondi (Reference 1) based on crystallographic data, gas kinetic collision cross sections, and liquid state properties . The 1 . Bondi, A ., J. Phys. Chem. 68, 441, 1964 . non-bonded contact distances predicted from the recommended 2 . Rowland, R . S . and Taylor, R ., J. Phys. Chem. 100, 7384, 1996 . 3 . Cambridge Crystallographic Data Center, www .ccdc .cam .ac .uk/prod- r of Bondi have been compared with actual data in the collec- vdw ucts/csd/radii/ tion of the Cambridge Crystallographic Data Center by Rowland and Taylor (Reference 2) and modified slightly . -

The Symbols of the Chemical Elements
42 THE SYMBOLS OF THE CHEMICAL ELEMENTS DARRYL FRANCIS Sutton, Surrey, England [email protected] The names of the chemical elements have received a certain amount of attention in Word Wa s over the years. The very first issue of Word Ways in February 1968 presented a quiz on 20 transposed element names. Later articles have offered more extensive transpositions, trnsadditions, old names for some of the elements, elements in US placenames, and words composed solely of the element symbols, such as CoAgULaTe. In this article, I want to examine the symbols of the chemical elements as an ordered coUection of letters. Many earlier items in Word Ways have treated the typewriter (computer) keyboard as an ordered sequence of letters (QWERTYillOPASDFGHJKLZXCVBNM) and have posed ques tions such as: • What is the longest word with its letters spelled in keyboard order? • What is the longest word with its letter spelled in rever e keyboard order? • What is the longest word with letters from the first letter row? Similar questions can be raised with regard to the elemental symbols. First off let's take a look at the periodic table, the listing of chemical elements in atomic number order and the corre ponding symbols. The list below contains 109 elements, with atomic numbers from 1 to 109. For three f the elements (aluminum, sulfur, cesium) there exist variant Briti h pellings (aluminium ulphur, caesium). For elements 104 to 109 I have used the new provisional name rather than the earlier suggested names. My 1998 printing of the Merriam-Webster ollegiate Di tionary lOth editi n. -
![Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N](https://docslib.b-cdn.net/cover/8398/atomic-and-ionic-radii-of-elements-1-96-martinrahm-a-roald-hoffmann-a-and-n-668398.webp)
Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N
DOI:10.1002/chem.201602949 Full Paper & Elemental Radii Atomic and Ionic Radii of Elements 1–96 MartinRahm,*[a] Roald Hoffmann,*[a] and N. W. Ashcroft[b] Abstract: Atomic and cationic radii have been calculated for tive measureofthe sizes of non-interacting atoms, common- the first 96 elements, together with selected anionicradii. ly invoked in the rationalization of chemicalbonding, struc- The metric adopted is the average distance from the nucleus ture, and different properties. Remarkably,the atomic radii where the electron density falls to 0.001 electrons per bohr3, as defined in this way correlate well with van der Waals radii following earlier work by Boyd. Our radii are derived using derived from crystal structures. Arationalizationfor trends relativistic all-electron density functional theory calculations, and exceptionsinthose correlations is provided. close to the basis set limit. They offer asystematic quantita- Introduction cule,[2] but we prefer to follow through with aconsistent pic- ture, one of gauging the density in the atomic groundstate. What is the size of an atom or an ion?This question has been The attractivenessofdefining radii from the electron density anatural one to ask over the centurythat we have had good is that a) the electron density is, at least in principle, an experi- experimental metricinformation on atoms in every form of mental observable,and b) it is the electron density at the out- matter,and (more recently) reliable theory for thesesame ermost regionsofasystem that determines Pauli/exchange/ atoms. And the momentone asks this question one knows same-spinrepulsions, or attractive bondinginteractions, with that there is no unique answer.Anatom or ion coursing down achemical surrounding. -

ARC: an Open-Source Library for Calculating Properties of Alkali Rydberg Atoms
ARC: An open-source library for calculating properties of alkali Rydberg atoms N. Šibalic´a,∗, J. D. Pritchardb, C. S. Adamsa, K. J. Weatherilla aJoint Quantum Center (JQC) Durham-Newcastle, Department of Physics, Durham University, South Road, Durham, DH1 3LE, United Kingdom bDepartment of Physics, SUPA, University of Strathclyde, 107 Rottenrow East, Glasgow, G4 0NG, United Kingdom Abstract We present an object-oriented Python library for computation of properties of highly-excited Rydberg states of alkali atoms. These include single-body effects such as dipole matrix elements, excited-state lifetimes (radiative and black-body limited) and Stark maps of atoms in external electric fields, as well as two-atom interaction potentials accounting for dipole and quadrupole coupling effects valid at both long and short range for arbitrary placement of the atomic dipoles. The package is cross-referenced to precise measurements of atomic energy levels and features extensive documentation to facilitate rapid upgrade or expansion by users. This library has direct application in the field of quantum information and quantum optics which exploit the strong Rydberg dipolar interactions for two-qubit gates, robust atom-light interfaces and simulating quantum many-body physics, as well as the field of metrology using Rydberg atoms as precise microwave electrometers. Keywords: Alkali atom, Matrix elements, Dipole-dipole interactions, Stark shift, Förster resonances PROGRAM SUMMARY They are a flourishing field for quantum information process- Program Title: ARC: Alkali Rydberg Calculator ing [1, 2] and quantum optics [3, 4, 5] in the few to single exci- Licensing provisions: BSD-3-Clause tation regime, as well as many-body physics [6, 7, 8, 9, 10, 11], Programming language: Python 2.7 or 3.5, with C extension in the many-excitations limit. -

Adverse Health Effects of Heavy Metals in Children
TRAINING FOR HEALTH CARE PROVIDERS [Date …Place …Event …Sponsor …Organizer] ADVERSE HEALTH EFFECTS OF HEAVY METALS IN CHILDREN Children's Health and the Environment WHO Training Package for the Health Sector World Health Organization www.who.int/ceh October 2011 1 <<NOTE TO USER: Please add details of the date, time, place and sponsorship of the meeting for which you are using this presentation in the space indicated.>> <<NOTE TO USER: This is a large set of slides from which the presenter should select the most relevant ones to use in a specific presentation. These slides cover many facets of the problem. Present only those slides that apply most directly to the local situation in the region. Please replace the examples, data, pictures and case studies with ones that are relevant to your situation.>> <<NOTE TO USER: This slide set discusses routes of exposure, adverse health effects and case studies from environmental exposure to heavy metals, other than lead and mercury, please go to the modules on lead and mercury for more information on those. Please refer to other modules (e.g. water, neurodevelopment, biomonitoring, environmental and developmental origins of disease) for complementary information>> Children and heavy metals LEARNING OBJECTIVES To define the spectrum of heavy metals (others than lead and mercury) with adverse effects on human health To describe the epidemiology of adverse effects of heavy metals (Arsenic, Cadmium, Copper and Thallium) in children To describe sources and routes of exposure of children to those heavy metals To understand the mechanism and illustrate the clinical effects of heavy metals’ toxicity To discuss the strategy of prevention of heavy metals’ adverse effects 2 The scope of this module is to provide an overview of the public health impact, adverse health effects, epidemiology, mechanism of action and prevention of heavy metals (other than lead and mercury) toxicity in children. -

THULIUM Element Symbol: Tm Atomic Number: 69
THULIUM Element Symbol: Tm Atomic Number: 69 An initiative of IYC 2011 brought to you by the RACI IONA JOHNSON www.raci.org.au THULIUM Element symbol: Tm Atomic number: 69 The element Thulium (when purified from mineral ores) is a silver-grey lustrous metal. Thulium metal is soft, malleable and ductile, and is so soft that it can be cut with a knife. The density is 9.32 grams per cubic centimetre: which is a bit lighter than Lead, but heavier than Iron, Copper, Nickel, and Tin. The surface of the metal will readily tarnish in air and produce an oxide. Thulium will also burn readily in air. Thulium will react slowly with cold water, and quite quickly with hot water to form Thulium hydroxide and Hydrogen. Thulium was named in honour of Thule: an ancient Roman name for a mythical country in the far North, which was probably Scandinavia. The first compound containing Thulium was discovered and named by Swedish Chemist Per Teodor Cleve (1840 – 1905) in 1879. Cleve made his discovery while studying the black-coloured rock that had been discovered around the town of Ytterby, Sweden in 1787. After removing all the other components from the rock the most interesting portion was where the Thulium accumulated because the solution possessed a bluish green colour. The compound was Thulium oxide (also called Thulia). Solid Thulium oxide has a pale green colour. The complete analysis of that rock took more than 100 years, and in the process nine new elements were discovered including Thulium. Pure metallic Thulium was not produced until 1910 by Charles James (1880-1928) an American chemist. -

Chalcogen-Nitrogen Bond: Insights Into a Key Chemical Motif
Proceedings Chalcogen-nitrogen Bond: Insights into A Key Chemical Motif Marco Bortoli,1 Andrea Madabeni,1 Pablo Andrei Nogara,2 Folorunsho B. Omage,2 Giovanni Ribaudo,3 Davide Zeppilli,1 Joao Batista Teixeira Rocha,2* Laura Orian1* 1 Dipartimento di Scienze Chimiche Università degli Studi di Padova Via Marzolo 1 35131 Padova, Italy; [email protected] (M.B.); [email protected] (A.M..); [email protected] (D.Z.) 2 Departamento de Bioquímica e Biologia Molecular, Universidade Federal de Santa Maria, Santa Maria, 97105-900, RS Brazil; [email protected] (P.A.N.); [email protected] (F.B.O.) 3 Dipartimento di Medicina Molecolare e Traslazionale, Università degli Studi di Brescia, Viale Europa 11, 25123 Brescia, Italy; [email protected] (G.R.) * Correspondence: : [email protected] (J.B.T.R), [email protected] (L.O.); † Presented at the 1st International Electronic Conference on Catalysis Sciences, 10–30 November 2020; Available online: https://eccs2020.sciforum.net/ Published: 10 November 2020 Abstract: Chalcogen-nitrogen chemistry deals with systems in which sulfur, selenium or tellurium is linked to a nitrogen nucleus. This chemical motif is a key component of different functional structures, ranging from inorganic materials and polymers to rationally designed catalysts, to bioinspired molecules and enzymes. The formation of a selenium-nitrogen bond, and its disruption, are rather common events in organic Se-catalyzed processes. In nature, along the mechanistic path of glutathione peroxidase, evidence of the formation of a Se-N bond in highly oxidizing conditions has been reported and interpreted as a strategy to protect the selenoenzyme from overoxidation.