New Alien Crayfish Species in Central Europe
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CRAYFISH IDENTIFICATION with DICHOTOMOUS KEYS As The
CRAYFISH IDENTIFICATION WITH DICHOTOMOUS KEYS As the name implies, a dichotomous key includes a series of paired choices called couplets. Each couplet contains alternative identification characteristics that allow you to advance step by step through the key, until you reach an endpoint (species). Once you reach your endpoint, read through the associated species account, and review the range map to make sure it fits what you found. If after reviewing the account and the map, you think you reached the species in error, go back to the key, and think about where you had to make a difficult decision on which way to proceed in the key. Then, try the opposite choice to see if it leads you to a better answer. When starting out, using dichotomous keys can be frustrating. Sometimes the characteristics presented in the key are straightforward, while other times the differences are extremely subtle. Seldom will every characteristic presented in a couplet match your specimen exactly. Be patient, choose the “best” answer, and as mentioned above, be ready to try alternative routes within the key. Keep in mind also that the photo included within a given species account represents a single, clean individual. There will often be variability in color and size depending on the sex and age class of the individual you are attempting to identify. You will see a good example of this when you are using one of the keys where Cambarus latimanus is present. The common name of this species is Variable Crayfish, and the name is fitting. This species is so variable that in some keys it appears in two places. -

DNA-Based Methods for Freshwater Biodiversity Conservation
DNA-based methods for freshwater biodiversity conservation - Phylogeographic analysis of noble crayfish (Astacus astacus) and new insights into the distribution of crayfish plague DISSERTATION zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften Fachbereich 7: Natur- und Umweltwissenschaften der Universität Koblenz-Landau Campus Landau vorgelegt am 16. Januar 2013 von Anne Schrimpf geboren am 21. September 1984 in Frankfurt am Main Referent: Prof. Dr. Ralf Schulz Koreferent: Prof. Dr. Klaus Schwenk - This thesis is dedicated to my grandparents - Content CONTENT CONTENT ............................................................................................................... 5 ABSTRACT ............................................................................................................ 8 ZUSAMMENFASSUNG ........................................................................................ 10 ABBEREVIATIONS .............................................................................................. 13 GENERAL INTRODUCTION ................................................................................ 15 Conservation of biological diversity ........................................................................ 15 The freshwater crayfish ............................................................................................ 17 General ............................................................................................................... 17 The noble crayfish (Astacus astacus) ................................................................ -

FINAL REPORT Status Survey for Three Rare Alabama Crayfishes
FINAL REPORT Status Survey for three rare Alabama crayfishes, Cambarus cracens, Cambarus scotti, and Cambarus unestami ILLINOIS NATURAL HISTORY SURVEY PRAIRIE RESEARCH INSTITUTE UNIVERSITY OF ILLINOIS AT URBANA CHAMPAIGN 1816 S. OAK CHAMPAIGN, IL 61820 INHS TECHNICAL REPORT 2012(21) BY Stephanie L. Kilburn Illinois Natural History Survey 1816 S. Oak Champaign, IL 61820 Christopher A. Taylor, Ph. D. Illinois Natural History Survey 1816 S. Oak Champaign, IL 61820 AND Guenter A. Schuster, Ph.D. 305 Boone Way Richmond, KY 40475 PREPARED FOR: U.S. Department of the Interior U.S. Fish and Wildlife Service Alabama Ecological Services Field Office 1208-B Main Street Daphne, AL 36526 20 July 2012 Introduction The Southeastern United States is famous for aquatic biodiversity. This area is known as a hotspot for fish and mussel species and is the most diverse region in the world for freshwater crayfishes. Because of this, the region is also an area of great conservation concern. A review by Taylor et al. (2007) found that nearly half of the crayfish in the area were in need of some conservation attention. This is of particular importance for the state of Alabama and its 85 species of crayfish, some of which are limited to a single drainage and are still substantially understudied. Three such species were the focus of the current study. The Slender Claw crayfish, Cambarus cracens, the Chattooga River Crayfish, C. scotti, and the Blackbarred Crayfish, C. unestami each have limited ranges confined to northeastern Alabama and northwestern Georgia. As such they are vulnerable to population declines due to single catastrophic events and are listed as either Endangered (C. -

Imports of Ornamental Crayfish
Knowledge and Management of Aquatic Ecosystems (2015) 416, 04 http://www.kmae-journal.org c ONEMA, 2015 DOI: 10.1051/kmae/2014040 Imports of ornamental crayfish: the first decade from the Czech Republic’s perspective J. Patoka(1),L.Kalous(1),, O. Kopecký(1) Received October 6, 2014 Revised December 18, 2014 Accepted December 21, 2014 ABSTRACT Key-words: The import of aquarium animals has been increasing worldwide in recent pet trade, years. Despite its contribution to world trade and the economy, this trade aquarium, also comprises one of the main pathways for the introduction of non- crustaceans, indigenous animals. In the past decade, crayfish has become a popular non-indigenous pet as well as a potential threat to the environment upon its escape or species, release. Since the Czech Republic is one of the world’s leading importer, price exporter, and producer of aquatic ornamental animals, we prepared a de- tailed analysis of crayfish imports. The present paper provides a complete list of countries supplying ornamental crayfish and examines trends of their prices and imported quantities during the past decade (2003–2012). Indonesia has been identified as the leading supplier in recent years. The annual average price of imported crayfish has varied over the evaluated period within the range of c0.76–4.72 per individual and it is rising annu- ally by c0.15. The quantity of live crayfish imported for aquarium purposes has not been affected significantly by the price per individual and it has grown rapidly. Therefore a constant monitoring of this pet trade sector is strongly recommended for the future. -

Estimating the Threat Posed by the Crayfish Plague Agent
Estimating the threat posed by the crayfish plague agent Aphanomyces astaci to crayfish species of Europe and North America — Introduction pathways, distribution and genetic diversity by Jörn Panteleit from Aachen, Germany Accepted Dissertation thesis for the partial fulfillment of the requirements for a Doctor of Natural Sciences Fachbereich 7: Natur- und Umweltwissenschaften Universität Koblenz-Landau Thesis examiners: Prof. Dr. Ralf Schulz, University of Koblenz-Landau, Germany Dr. Japo Jussila, University of Eastern Finland, Kuopio, Suomi-Finland Date of oral examination: January 17th, 2019 TABLE OF CONTENTS 1. LIST OF PUBLICATIONS ........................................................................................................................ 3 2. ABSTRACT ............................................................................................................................................ 4 2.1 Zusammenfassung ......................................................................................................................... 5 3. ABBREVIATIONS .................................................................................................................................. 6 4. INTRODUCTION ................................................................................................................................... 7 4.1 Invasive species ............................................................................................................................. 7 4.2 Freshwater crayfish in Europe ...................................................................................................... -

Crayfishes and Shrimps) of Arkansas with a Discussion of Their Ah Bitats Raymond W
Journal of the Arkansas Academy of Science Volume 34 Article 9 1980 Inventory of the Decapod Crustaceans (Crayfishes and Shrimps) of Arkansas with a Discussion of Their aH bitats Raymond W. Bouchard Southern Arkansas University Henry W. Robison Southern Arkansas University Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Terrestrial and Aquatic Ecology Commons Recommended Citation Bouchard, Raymond W. and Robison, Henry W. (1980) "Inventory of the Decapod Crustaceans (Crayfishes and Shrimps) of Arkansas with a Discussion of Their aH bitats," Journal of the Arkansas Academy of Science: Vol. 34 , Article 9. Available at: http://scholarworks.uark.edu/jaas/vol34/iss1/9 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Journal of the Arkansas Academy of Science, Vol. 34 [1980], Art. 9 AN INVENTORY OF THE DECAPOD CRUSTACEANS (CRAYFISHES AND SHRIMPS) OF ARKANSAS WITH A DISCUSSION OF THEIR HABITATS i RAYMOND W. BOUCHARD 7500 Seaview Avenue, Wildwood Crest, New Jersey 08260 HENRY W. ROBISON Department of Biological Sciences Southern Arkansas University, Magnolia, Arkansas 71753 ABSTRACT The freshwater decapod crustaceans of Arkansas presently consist of two species of shrimps and 51 taxa of crayfishes divided into 47 species and four subspecies. -

Decapoda: Cambaridae) of Arkansas Henry W
Journal of the Arkansas Academy of Science Volume 71 Article 9 2017 An Annotated Checklist of the Crayfishes (Decapoda: Cambaridae) of Arkansas Henry W. Robison Retired, [email protected] Keith A. Crandall George Washington University, [email protected] Chris T. McAllister Eastern Oklahoma State College, [email protected] Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Robison, Henry W.; Crandall, Keith A.; and McAllister, Chris T. (2017) "An Annotated Checklist of the Crayfishes (Decapoda: Cambaridae) of Arkansas," Journal of the Arkansas Academy of Science: Vol. 71 , Article 9. Available at: http://scholarworks.uark.edu/jaas/vol71/iss1/9 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. An Annotated Checklist of the Crayfishes (Decapoda: Cambaridae) of Arkansas Cover Page Footnote Our deepest thanks go to HWR’s numerous former SAU students who traveled with him in search of crayfishes on many fieldtrips throughout Arkansas from 1971 to 2008. Personnel especially integral to this study were C. -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Arrival of Non-Indigenous Crayfish Species
Knowl. Manag. Aquat. Ecosyst. 2016, 417, 37 Knowledge & © G. Kotovska et al., Published by EDP Sciences 2016 Management of Aquatic DOI: 10.1051/kmae/2016024 Ecosystems www.kmae-journal.org Journal fully supported by Onema RESEARCH PAPER East European crayfish stocks at risk: arrival of non-indigenous crayfish species Ganna Kotovska1, Dmytro Khrystenko1,2,*,Jiří Patoka3,a and Antonín Kouba4,a 1 Institute of Fisheries of the National Academy of Agrarian Sciences of Ukraine, Obuhivska 135, Kyiv 03164, Ukraine 2 Cornell University, College of Agriculture and Life Sciences, International Programs, B75 Mann Library, Ithaca, NY 14853, USA 3 Czech University of Life Sciences Prague, Faculty of Agrobiology, Food and Natural Resources, Department of Zoology and Fisheries, Kamycká 129, Prague 6–Suchdol 165 21, Czech Republic 4 University of South Bohemia in České Budějovice, Faculty of Fisheries and Protection of Waters, South Bohemian Research Center of Aquaculture and Biodiversity of Hydrocenoses, Zátisí 728/II, Vodňany 389 25, Czech Republic Abstract – An increasing number of non-indigenous crayfish species (NICS) of apparently pet trade origin have become established particularly in Europe. Especially alarming are recent confirmation of two distantly separated marbled crayfish Procambarus fallax f. virginalis populations in Ukraine and indications of more North American cambarids present in the local pet market. The present study aimed to investigate crayfish species availability within the Ukrainian pet trade together with the climate match and risk they represent to the freshwater ecosystems generally and indigenous crayfish species in particular. Altogether, 15 NICS belonging to all three crayfish families were detected. Considering their origin, availability, probability of establishment, invasiveness and further aspects, marbled crayfish and red swamp crayfish Procambarus clarkii appear to be potentially the most troubling. -

Fish-Game "Conservation of Wildlife Through Education"
CALIFORNIA! FISH-GAME "CONSERVATION OF WILDLIFE THROUGH EDUCATION" I VOLUME 69 of wild- California Fish and Came is a journal devoted to the conservation the California life. If its contents are reproduced elsewhere, the authors and Department of Fish and Game would appreciate being acknowledged. an Subscriptions may be obtained at the rate of $5 per year by placing order with the California Department of Fish and Game, 1416 Ninth Street, Sacramento, California 95814. Money orders and checks should be made out sub- to California Department of Fish and Game. Inquiries regarding paid scriptions should be directed to the Editor. Complimentary subscriptions are granted, on a limited basis, to libraries, scientific and educational institutions, conservation agencies, and on exchange. Complimentary subscriptions must be renewed annually by returning the post- card enclosed with each October issue. Please direct correspondence to: Perry L. Herrgesell, Ph.D., Editor California Fish and Game 1416 Ninth Street Sacramento, California 95814 u D V VOLUME 69 OCTOBER 1983 NUMBER 4 Published Quarterly by STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME A —LDA— 194 CALIFORNIA FISH AND GAME STATE OF CALIFORNIA GEORGE DEUKMEJIAN, Governor THE RESOURCES AGENCY GORDON VAN VLECK, Secretary for Resources FISH AND GAME COMMISSION NORMAN B. UVERMORE, JR., President San Rafael WILLIAM A. BURKE, Ed.D., Vice President ABEL C. GALLETTI, Member Los Angeles Los Angeles BRIAN J. KAHN, Member ALBERT C. TAUCHER, Member Santa Rosa Long Beach DEPARTMENT OF FISH AND GAME HOWARD D. CARPER, Director 1416 9th Street Sacramento 95814 CALIFORNIA FISH AND GAME Editorial Staff Editorial staff for this issue consisted of the following: Wildlife Kenneth A. -
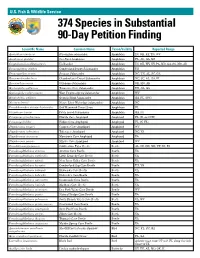
374 Species in Substantial 90-Day Petition Finding
U.S. Fish & Wildlife Service 374 Species in Substantial 90-Day Petition Finding Scientific Name Common Name Taxon/Validity Reported Range Ambystoma barbouri Streamside salamander Amphibian IN, OH, KY, TN, WV Amphiuma pholeter One-Toed Amphiuma Amphibian FL, AL, GA, MS Cryptobranchus alleganiensis Hellbender Amphibian IN, OH, WV, NY, PA, MD, GA, SC, MO, AR Desmognathus abditus Cumberland Dusky Salamander Amphibian TN Desmognathus aeneus Seepage Salamander Amphibian NC, TN, AL, SC, GA Eurycea chamberlaini Chamberlain's Dwarf Salamander Amphibian NC, SC, AL, GA, FL Eurycea tynerensis Oklahoma Salamander Amphibian OK, MO, AR Gyrinophilus palleucus Tennessee Cave Salamander Amphibian TN, AL, GA Gyrinophilus subterraneus West Virginia Spring Salamander Amphibian WV Haideotriton wallacei Georgia Blind Salamander Amphibian GA, FL (NW) Necturus lewisi Neuse River Waterdog (salamander) Amphibian NC Pseudobranchus striatus lustricolus Gulf Hammock Dwarf Siren Amphibian FL Urspelerpes brucei Patch-nosed Salamander Amphibian GA, SC Crangonyx grandimanus Florida Cave Amphipod Amphipod FL (N. and NW) Crangonyx hobbsi Hobb's Cave Amphipod Amphipod FL (N. FL) Stygobromus cooperi Cooper's Cave Amphipod Amphipod WV Stygobromus indentatus Tidewater Amphipod Amphipod NC, VA Stygobromus morrisoni Morrison's Cave Amphipod Amphipod VA Stygobromus parvus Minute Cave Amphipod Amphipod WV Cicindela marginipennis Cobblestone Tiger Beetle Beetle AL, IN, OH, NH, VT, NJ, PA Pseudanophthalmus avernus Avernus Cave Beetle Beetle VA Pseudanophthalmus cordicollis Little -

Threatened and Endangered Species List
Effective April 15, 2009 - List is subject to revision For a complete list of Tennessee's Rare and Endangered Species, visit the Natural Areas website at http://tennessee.gov/environment/na/ Aquatic and Semi-aquatic Plants and Aquatic Animals with Protected Status State Federal Type Class Order Scientific Name Common Name Status Status Habit Amphibian Amphibia Anura Gyrinophilus gulolineatus Berry Cave Salamander T Amphibian Amphibia Anura Gyrinophilus palleucus Tennessee Cave Salamander T Crustacean Malacostraca Decapoda Cambarus bouchardi Big South Fork Crayfish E Crustacean Malacostraca Decapoda Cambarus cymatilis A Crayfish E Crustacean Malacostraca Decapoda Cambarus deweesae Valley Flame Crayfish E Crustacean Malacostraca Decapoda Cambarus extraneus Chickamauga Crayfish T Crustacean Malacostraca Decapoda Cambarus obeyensis Obey Crayfish T Crustacean Malacostraca Decapoda Cambarus pristinus A Crayfish E Crustacean Malacostraca Decapoda Cambarus williami "Brawley's Fork Crayfish" E Crustacean Malacostraca Decapoda Fallicambarus hortoni Hatchie Burrowing Crayfish E Crustacean Malocostraca Decapoda Orconectes incomptus Tennessee Cave Crayfish E Crustacean Malocostraca Decapoda Orconectes shoupi Nashville Crayfish E LE Crustacean Malocostraca Decapoda Orconectes wrighti A Crayfish E Fern and Fern Ally Filicopsida Polypodiales Dryopteris carthusiana Spinulose Shield Fern T Bogs Fern and Fern Ally Filicopsida Polypodiales Dryopteris cristata Crested Shield-Fern T FACW, OBL, Bogs Fern and Fern Ally Filicopsida Polypodiales Trichomanes boschianum