Ncomms5993.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2007/0254315 A1 Cox Et Al
US 20070254315A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0254315 A1 Cox et al. (43) Pub. Date: Nov. 1, 2007 (54) SCREENING FOR NEUROTOXIC AMINO (60) Provisional application No. 60/494.686, filed on Aug. ACID ASSOCATED WITH NEUROLOGICAL 12, 2003. DSORDERS Publication Classification (75) Inventors: Paul A. Cox, Provo, UT (US); Sandra A. Banack, Fullerton, CA (US); Susan (51) Int. Cl. J. Murch, Cambridge (CA) GOIN 33/566 (2006.01) GOIN 33/567 (2006.01) Correspondence Address: (52) U.S. Cl. ............................................................ 435/721 PILLSBURY WINTHROP SHAW PITTMAN LLP (57) ABSTRACT ATTENTION: DOCKETING DEPARTMENT Methods for screening for neurological disorders are dis P.O BOX 105OO closed. Specifically, methods are disclosed for screening for McLean, VA 22102 (US) neurological disorders in a Subject by analyzing a tissue sample obtained from the subject for the presence of (73) Assignee: THE INSTITUTE FOR ETHNO elevated levels of neurotoxic amino acids or neurotoxic MEDICINE, Provo, UT derivatives thereof associated with neurological disorders. In particular, methods are disclosed for diagnosing a neu (21) Appl. No.: 11/760,668 rological disorder in a subject, or predicting the likelihood of developing a neurological disorder in a Subject, by deter (22) Filed: Jun. 8, 2007 mining the levels of B-N-methylamino-L-alanine (BMAA) Related U.S. Application Data in a tissue sample obtained from the subject. Methods for screening for environmental factors associated with neuro (63) Continuation of application No. 10/731,411, filed on logical disorders are disclosed. Methods for inhibiting, treat Dec. 8, 2003, now Pat. No. 7,256,002. -

Peripheral Neuropathy in Complex Inherited Diseases: an Approach To
PERIPHERAL NEUROPATHY IN COMPLEX INHERITED DISEASES: AN APPROACH TO DIAGNOSIS Rossor AM1*, Carr AS1*, Devine H1, Chandrashekar H2, Pelayo-Negro AL1, Pareyson D3, Shy ME4, Scherer SS5, Reilly MM1. 1. MRC Centre for Neuromuscular Diseases, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, London, WC1N 3BG, UK. 2. Lysholm Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, London, WC1N 3BG, UK. 3. Unit of Neurological Rare Diseases of Adulthood, Carlo Besta Neurological Institute IRCCS Foundation, Milan, Italy. 4. Department of Neurology, University of Iowa, 200 Hawkins Drive, Iowa City, IA 52242, USA 5. Department of Neurology, University of Pennsylvania, Philadelphia, PA 19014, USA. * These authors contributed equally to this work Corresponding author: Mary M Reilly Address: MRC Centre for Neuromuscular Diseases, 8-11 Queen Square, London, WC1N 3BG, UK. Email: [email protected] Telephone: 0044 (0) 203 456 7890 Word count: 4825 ABSTRACT Peripheral neuropathy is a common finding in patients with complex inherited neurological diseases and may be subclinical or a major component of the phenotype. This review aims to provide a clinical approach to the diagnosis of this complex group of patients by addressing key questions including the predominant neurological syndrome associated with the neuropathy e.g. spasticity, the type of neuropathy, and the other neurological and non- neurological features of the syndrome. Priority is given to the diagnosis of treatable conditions. Using this approach, we associated neuropathy with one of three major syndromic categories - 1) ataxia, 2) spasticity, and 3) global neurodevelopmental impairment. Syndromes that do not fall easily into one of these three categories can be grouped according to the predominant system involved in addition to the neuropathy e.g. -
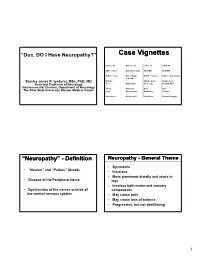
Case Vignettes
“Doc, DO I Have Neuropathy?” Case Vignettes Susan, 50 Gwenn, 32 John, 52 Sally, 42 DM – 12 yrs Hypothyroidism No PMH No PMH B/N/T – 2 yrs N/T at night B/N/T – 6 days B/N/T – few months 3 months Stanley Jones P. Iyadurai, MSc, PhD, MD Hands, Hands, Arms Hands, Feet Assistant Professor of Neurology Feet Right hand Feet, Legs Burning Pain Neuromuscular Division, Department of Neurology Arms, Difficulty Pain Pain The Ohio State University Wexner Medical Center Legs Opening jars Breathing Redness Imbalance Normal gait Imbalance Normal Strength “Neuropathy” - Definition Neuropathy - General Theme • Symmetric • “Neuron” and “Pathos” (Greek) • Insidious • More prominent distally and starts in • Disease of the Peripheral Nerve legs • Involves both motor and sensory • Dysfunction of the nerves outside of components the central nervous system • May cause pain • May cause loss of balance • Progressive, but not debilitating 1 Not all that tingles is Classification - Functional neuropathy ‘numbness and tingling’ • Motor – affects the motor nerves Weakness • not all numbness and tingling is • Sensory peripheral nerve ‒ Pain • RLS ‒ Small Fiber ‒ Large Fiber • edema ‒ Large and Small Fiber • deconditioning ‒ Neuronopathy (ganglionopathy) • central nervous system • Autonomic • Sweating Changes, Blood Pressure Changes • nerve root [‘sciatica’] Classification – Time-course Classification – Time-course • Acute Immune-mediated • Congenital/Hereditary • Infantile Weakness • Childhood-onset • Acquired • Relapsing ‒ Reversible? • Hereditary ‒ Demyelinating? -

Ataxia with Loss of Purkinje Cells in a Mouse Model for Refsum Disease
Ataxia with loss of Purkinje cells in a mouse model for Refsum disease Sacha Ferdinandussea,1,2, Anna W. M. Zomerb,1, Jasper C. Komena, Christina E. van den Brinkb, Melissa Thanosa, Frank P. T. Hamersc, Ronald J. A. Wandersa,d, Paul T. van der Saagb, Bwee Tien Poll-Thed, and Pedro Britesa Academic Medical Center, Departments of aClinical Chemistry (Laboratory of Genetic Metabolic Diseases) and dPediatrics, Emma’s Children Hospital, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; bHubrecht Institute, Royal Netherlands Academy of Arts and Sciences 3584 CT Utrecht, The Netherlands; and cRehabilitation Hospital ‘‘De Hoogstraat’’ Rudolf Magnus Institute of Neuroscience, 3584 CG Utrecht, The Netherlands Edited by P. Borst, The Netherlands Cancer Institute, Amsterdam, The Netherlands, and approved October 3, 2008 (received for review June 23, 2008) Refsum disease is caused by a deficiency of phytanoyl-CoA hy- Clinically, Refsum disease is characterized by cerebellar droxylase (PHYH), the first enzyme of the peroxisomal ␣-oxidation ataxia, polyneuropathy, and progressive retinitis pigmentosa, system, resulting in the accumulation of the branched-chain fatty culminating in blindness, (1, 3). The age of onset of the symptoms acid phytanic acid. The main clinical symptoms are polyneuropathy, can vary from early childhood to the third or fourth decade of cerebellar ataxia, and retinitis pigmentosa. To study the patho- life. No treatment is available for patients with Refsum disease, genesis of Refsum disease, we generated and characterized a Phyh but they benefit from a low phytanic acid diet. Phytanic acid is knockout mouse. We studied the pathological effects of phytanic derived from dietary sources only, specifically from the chloro- acid accumulation in Phyh؊/؊ mice fed a diet supplemented with phyll component phytol. -

ICD9 & ICD10 Neuromuscular Codes
ICD-9-CM and ICD-10-CM NEUROMUSCULAR DIAGNOSIS CODES ICD-9-CM ICD-10-CM Focal Neuropathy Mononeuropathy G56.00 Carpal tunnel syndrome, unspecified Carpal tunnel syndrome 354.00 G56.00 upper limb Other lesions of median nerve, Other median nerve lesion 354.10 G56.10 unspecified upper limb Lesion of ulnar nerve, unspecified Lesion of ulnar nerve 354.20 G56.20 upper limb Lesion of radial nerve, unspecified Lesion of radial nerve 354.30 G56.30 upper limb Lesion of sciatic nerve, unspecified Sciatic nerve lesion (Piriformis syndrome) 355.00 G57.00 lower limb Meralgia paresthetica, unspecified Meralgia paresthetica 355.10 G57.10 lower limb Lesion of lateral popiteal nerve, Peroneal nerve (lesion of lateral popiteal nerve) 355.30 G57.30 unspecified lower limb Tarsal tunnel syndrome, unspecified Tarsal tunnel syndrome 355.50 G57.50 lower limb Plexus Brachial plexus lesion 353.00 Brachial plexus disorders G54.0 Brachial neuralgia (or radiculitis NOS) 723.40 Radiculopathy, cervical region M54.12 Radiculopathy, cervicothoracic region M54.13 Thoracic outlet syndrome (Thoracic root Thoracic root disorders, not elsewhere 353.00 G54.3 lesions, not elsewhere classified) classified Lumbosacral plexus lesion 353.10 Lumbosacral plexus disorders G54.1 Neuralgic amyotrophy 353.50 Neuralgic amyotrophy G54.5 Root Cervical radiculopathy (Intervertebral disc Cervical disc disorder with myelopathy, 722.71 M50.00 disorder with myelopathy, cervical region) unspecified cervical region Lumbosacral root lesions (Degeneration of Other intervertebral disc degeneration, -
Come to Your Senses: Atypical Polyneuropathy
Come To Your Senses: Atypical Polyneuropathy Damian Campbell, DO; Joshua Lovell, DO; Krishna Pokala, MD; Yessar Hussain, MD The University of Texas at Austin, Department of Neurology, Dell Medical School Clinical History Workup Diagnosis and Discussion Pathophysiology 35 year old female was referred to the neurology clinic for evaluation of an Electrodiagnostics Our patients clinical history and genetic testing results were consistent and There is a great deal of variability in the presentation of symptoms; as there is abnormal gait. She reported slow but progressive changes in her gait over the she was diagnosed with PHARC: Polyneuropathy, Hearing Loss, Ataxia, variability amongst the gene mutations leading to inactive ABHD12. Despite the course of the last ten years. Her changes ranged from a sense of feeling off- Nerve Conductions Retinitis Pigmentosa, Cataracts. variability that exists within gene mutations, it is posited that all of the balanced to shooting pain down each leg She felt that her pain originated in her Distal Latency Peak Amplitude Conduction Velocity homozygous mutations result in complete loss-of-function of the ABHD12 low back and radiated posteriorly down each leg without any accompanying Latency (m/sec) PHARC was first described by Fiskerstrand et al in 2008.(1) At that time they enzyme. Reduction in ABHD12 results in increased levels of endocannabinoid Right Median CMAP 4.3 7.8 numbness, weakness, or bowel and bladder involvement. Of significance is her were attempting to genetically characterize a neurological disorder that arachidonoyl glycerol 2-AG, which has important functions in synaptic plasticity 11.6 5.8 26 resembled Refsum disease in a Norwegian family. -

Retinitis Pigmentosa, Ataxia, and Peripheral Neuropathy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.46.3.206 on 1 March 1983. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1983;46:206-213 Retinitis pigmentosa, ataxia, and peripheral neuropathy RR TUCK, JG McLEOD From the Department ofMedicine, University ofSydney, Australia SUMMARY The clinical features of four patients with retinitis pigmentosa, ataxia and peripheral neuropathy but with no increase in serum phytanic acid are reported. Three patients also had sensorineural deafness and radiological evidence of cerebellar atrophy. Nerve conduction studies revealed abnormalities of sensory conduction and normal or only mild slowing of motor conduc- tion velocity. Sural nerve biopsy demonstrated a reduction in the density of myelinated fibres. There were no onion bulb formations. These cases clinically resemble Refsum's disease, but differ in having no detectable biochemical abnormality, and a peripheral neuropathy which is not hypertrophic in type. They may represent unusual cases of spinocerebellar degeneration. Retinitis pigmentosa occurs infrequently as an iso- (WAIS). He had a speech impediment but was not dysar- Protected by copyright. lated finding in otherwise healthy individuals and thric. He was of short stature, had a small head and pes families. Its association with deafness, with or with- cavus but no kyphoscoliosis. His visual acuity in the right eye was 6/60 while in the left he could count fingers only. out other neurological abnormalities is much less The right visual field was constricted but the left could not common but nevertheless well recognised.1 In be tested. The optic discs were pale, the retinal vessels heredopathia atactica polyneuritiformis (Refsum's small in diameter and throughout the retinae there was disease), abetalipoproteinaemia, and the Keams- scattered "bone corpuscle" pigmentation. -
A Dictionary of Neurological Signs
FM.qxd 9/28/05 11:10 PM Page i A DICTIONARY OF NEUROLOGICAL SIGNS SECOND EDITION FM.qxd 9/28/05 11:10 PM Page iii A DICTIONARY OF NEUROLOGICAL SIGNS SECOND EDITION A.J. LARNER MA, MD, MRCP(UK), DHMSA Consultant Neurologist Walton Centre for Neurology and Neurosurgery, Liverpool Honorary Lecturer in Neuroscience, University of Liverpool Society of Apothecaries’ Honorary Lecturer in the History of Medicine, University of Liverpool Liverpool, U.K. FM.qxd 9/28/05 11:10 PM Page iv A.J. Larner, MA, MD, MRCP(UK), DHMSA Walton Centre for Neurology and Neurosurgery Liverpool, UK Library of Congress Control Number: 2005927413 ISBN-10: 0-387-26214-8 ISBN-13: 978-0387-26214-7 Printed on acid-free paper. © 2006, 2001 Springer Science+Business Media, Inc. All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, Inc., 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dis- similar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to propri- etary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omis- sions that may be made. -

Visual Impairment Age-Related Macular
VISUAL IMPAIRMENT AGE-RELATED MACULAR DEGENERATION Macular degeneration is a medical condition predominantly found in young children in which the center of the inner lining of the eye, known as the macula area of the retina, suffers thickening, atrophy, and in some cases, watering. This can result in loss of side vision, which entails inability to see coarse details, to read, or to recognize faces. According to the American Academy of Ophthalmology, it is the leading cause of central vision loss (blindness) in the United States today for those under the age of twenty years. Although some macular dystrophies that affect younger individuals are sometimes referred to as macular degeneration, the term generally refers to age-related macular degeneration (AMD or ARMD). Age-related macular degeneration begins with characteristic yellow deposits in the macula (central area of the retina which provides detailed central vision, called fovea) called drusen between the retinal pigment epithelium and the underlying choroid. Most people with these early changes (referred to as age-related maculopathy) have good vision. People with drusen can go on to develop advanced AMD. The risk is considerably higher when the drusen are large and numerous and associated with disturbance in the pigmented cell layer under the macula. Recent research suggests that large and soft drusen are related to elevated cholesterol deposits and may respond to cholesterol lowering agents or the Rheo Procedure. Advanced AMD, which is responsible for profound vision loss, has two forms: dry and wet. Central geographic atrophy, the dry form of advanced AMD, results from atrophy to the retinal pigment epithelial layer below the retina, which causes vision loss through loss of photoreceptors (rods and cones) in the central part of the eye. -

Charcot-Marie-Tooth Disease and Other Genetic Polyneuropathies
Review Article 04/25/2018 on mAXWo3ZnzwrcFjDdvMDuzVysskaX4mZb8eYMgWVSPGPJOZ9l+mqFwgfuplwVY+jMyQlPQmIFeWtrhxj7jpeO+505hdQh14PDzV4LwkY42MCrzQCKIlw0d1O4YvrWMUvvHuYO4RRbviuuWR5DqyTbTk/icsrdbT0HfRYk7+ZAGvALtKGnuDXDohHaxFFu/7KNo26hIfzU/+BCy16w7w1bDw== by https://journals.lww.com/continuum from Downloaded Downloaded Address correspondence to Dr Sindhu Ramchandren, University of Michigan, from Charcot-Marie-Tooth Department of Neurology, https://journals.lww.com/continuum 2301 Commonwealth Blvd #1023, Ann Arbor, MI 48105, Disease and [email protected]. Relationship Disclosure: Dr Ramchandren has served Other Genetic on advisory boards for Biogen and Sarepta Therapeutics, by mAXWo3ZnzwrcFjDdvMDuzVysskaX4mZb8eYMgWVSPGPJOZ9l+mqFwgfuplwVY+jMyQlPQmIFeWtrhxj7jpeO+505hdQh14PDzV4LwkY42MCrzQCKIlw0d1O4YvrWMUvvHuYO4RRbviuuWR5DqyTbTk/icsrdbT0HfRYk7+ZAGvALtKGnuDXDohHaxFFu/7KNo26hIfzU/+BCy16w7w1bDw== Inc, and has received research/grant support from Polyneuropathies the Muscular Dystrophy Association (Foundation Sindhu Ramchandren, MD, MS Clinic Grant) and the National Institutes of Health (K23 NS072279). Unlabeled Use of ABSTRACT Products/Investigational Purpose of Review: Genetic polyneuropathies are rare and clinically heterogeneous. Use Disclosure: This article provides an overview of the clinical features, neurologic and electrodiagnostic Dr Ramchandren reports no disclosure. findings, and management strategies for Charcot-Marie-Tooth disease and other * 2017 American Academy genetic polyneuropathies as well as an algorithm for genetic testing. of Neurology. -

General Disease Finder
General Disease Finder This overview will help to fi nd neuromuscular disease patterns in the different sections Cushing’s disease, steroid myopathy; Addison’s disease, general muscle weakness Adrenal dysfunction Periodic paralysis Aldosteronism Tetanic muscles CN: VII AIDS Polyneuropathies: infl ammatory, immune mediated, treatment related Myopathies: infl ammatory, treatment related Neoplastic: lymphoma (direct invasion) Opportunistic infections: CMV, toxoplasmosis, Cryptococcus , HSV Candida, Varicella, Histoplasma, TBC, Aspergillus CMV polyradiculomyelopathy Herpes zoster radiculitis Syphilitic radiculopathy Treatment related: polyneuropathy/myopathy Ddl, ddC, foscarnet, isoniazid Zidovudine Polyneuropathy (distal, rarely proximal) Alcohol Mononeuropathy – radial nerve (compression) Myopathy Acute necrotizing myopathy and myoglobinuria Chronic proximal weakness Hypokalemic paralysis Myoglobinuria Compartment syndromes (prolonged compression) Familial amyloid polyneuropathies Amyloid Transthyretin Neuropathy Sensorimotor neuropathy Autonomic (continued) E.L. Feldman et al., Atlas of Neuromuscular Diseases, 299 DOI 10.1007/978-3-7091-1605-0, © Springer-Verlag Wien 2014 300 General Disease Finder Autonomic involvement Primary/secondary Apolipoprotein A-1 Polyneuropathy, painful, hearing loss Gelsolin type V, VII, and other CN Mild polyneuropathy Primary amyloidosis (AL) Deposition of immunoglobulin light chains in tissue Adrenal dysfunction Aldosteronism AIDS Alcoholism Amyloid Painful neuropathy Autonomic involvement Carpal tunnel syndrome -

Inborn Errors of Metabolism As a Cause of Neurological Disease in Adults: an Approach to Investigation
J Neurol Neurosurg Psychiatry 2000;69:5–12 5 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.69.1.5 on 1 July 2000. Downloaded from REVIEW Inborn errors of metabolism as a cause of neurological disease in adults: an approach to investigation R G F Gray, M A Preece, S H Green, W Whitehouse, J Winer, A Green In 1927 Archibald Garrod presented the Hux- LYSOSOMAL STORAGE DISEASES ley Lecture at Charing Cross Hospital1 Out of The lysosome is an intracellular organelle this lecture emerged the concept of an “inborn involved in the degradation of various complex error of metabolism” whereby an inherited lipids, glycoproteins, and mucopolysaccha- defect may lead to the accumulation in cells or rides. Defects in specific enzymes lead to the body fluids of a metabolite which in itself may accumulation of complex catabolic intermedi- predispose to disease. The disorders cited as ates. Although the process occurs in utero the examples were all adult onset disorders. age of onset of clinical symptoms can vary sub- Today there are over 200 known inborn stantially. Alleles are known which are associ- errors of metabolism; however, the vast major- ated with a milder, later onset phenotype. This ity of cases reported are of childhood onset may be related to the presence of significant (<16 years of age). In part this may reflect the residual functional enzyme activity resulting in fact that the paediatric forms of the disease are a lower rate of accumulation of the intermedi- more severe and hence more easily recognis- ate metabolite. The clinical symptoms of the able.