Ancient Events and Climate Adaptive Capacity Shaped Distinct Chloroplast Genetic Structure in the Oak Lineages Mengxiao Yan1,2, Ruibin Liu1,3, Ying Li1,4, Andrew L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Book CMU 5(2)
CMU. Journal (2006) Vol. 5(2) 169 Survey and Herbarium Specimens of Medicinal Vascular Flora of Doi Suthep-Pui Somporn Putiyanan1* and J.F. Maxwell2 1 Department of Pharmaceutical Science, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand 2 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand *Corresponding author. E-mail: [email protected] ABSTRACT The herbarium includes over 9,285 specimens from 238 families (270 fam. in the word) in medicinal plant herbarium, Faculty of Pharmacy, Chiang Mai University. From July 1987 to September 1991, a total of 2,044 species have been collected from Doi Suthep- Pui National Park, some of which are of considerable economic, medicinal and botanical interest. Vascular plants in this national park comprise of 193 of the 228 known families of vascular plants in Thailand, including a new family record for the flora of Thailand (Lardizabalaceae), eleven species new records for Thailand, three emended descriptions, two new combinations, and at least two species, with several others that are probably undescribed and new to science. The lowland, mostly disturbed forests up to 350-950 m. elevation, are of two deciduous facies, viz., dipterocarp-oak and mixed (former teak) forest. Elevations above this to the summit of Doi Suthep (c.1,620 m.) and Doi Pui (c.1,685 m.) are primary evergreen (monsoon) with some residual pine on some of the ridges. There is a distinct dry season (December-May) during which there are fires and many of the lowland species flower and fruit, many become leafless while in the evergreen areas, there is no specific flowering or fruiting season, that is, the phenologies of the plants in this habitat vary according to each species throughout the year. -

Bulletin of Natural History ®
FLORI'IDA MUSEUM BULLETIN OF NATURAL HISTORY ® A MIDDLE EOCENE FOSSIL PLANT ASSEMBLAGE (POWERS CLAY PIT) FROM WESTERN TENNESSEE DavidL. Dilcher and Terry A. Lott Vol. 45, No. 1, pp. 1-43 2005 UNIVERSITY OF FLORIDA GAINESVILLE - The FLORIDA MUSEUM OF NATURAL HiSTORY is Florida«'s state museum of natural history, dedicated to understanding, preser¥ingrand interpreting].biologica[1 diversity and culturafheritage. The BULLETIN OF THE FLORIDA- MUSEUM OF NATURAL HISTORY is a peer-reviewed publication thatpziblishes.the result5 of origifial reseafchin zodlogy, botany, paleontology, and archaeology. Address all inquiries t6 the Managing Editor ofthe Bulletin. Numbers,ofthe Bulletin,afe,published,at itregular intervals. Specific volumes are not'necessarily completed in anyone year. The end of a volume willl·be noted at the foot of the first page ofthe last issue in that volume. Richard Franz, Managing Editor Erika H. Simons, Production BulletinCommittee Richard Franz,,Chairperson Ann Cordell Sarah Fazenbaker Richard Hulbert WilliamMarquardt Susan Milbrath Irvy R. Quitmyer - Scott Robinson, Ex 01#cio Afember ISSN: 0071-6154 Publication Date: October 31,2005 Send communications concerning purchase or exchange of the publication and manustfipt queries to: Managing Editor of the BULLETIN Florida MuseumofNatural-History University offlorida PO Box 117800 Gainesville, FL 32611 -7800 U.S.A. Phone: 352-392-1721 Fax: 352-846-0287 e-mail: [email protected] A MIDDLE EOCENE FOSSIL PLANT ASSEMBLAGE (POWERS CLAY PIT) FROM WESTERN TENNESSEE David L. Dilcher and Terry A. Lottl ABSTRACT Plant megafossils are described, illustrated and discussed from Powers Clay Pit, occurring in the middle Eocene, Claiborne Group of the Mississippi Embayment in western Tennessee. -

Complete Chloroplast Genome Sequence and Phylogenetic Analysis of Quercus Bawanglingensis Huang, Li Et Xing, a Vulnerable Oak Tree in China
Article Complete Chloroplast Genome Sequence and Phylogenetic Analysis of Quercus bawanglingensis Huang, Li et Xing, a Vulnerable Oak Tree in China Xue Liu 1 , Er-Mei Chang 1, Jian-Feng Liu 1,* , Yue-Ning Huang 1, Ya Wang 1, Ning Yao 1 and Ze-Ping Jiang 1,2 1 Key Laboratory of Tree Breeding and Cultivation of State Forestry Administration, Research Institute of Forestry, Chinese Academy of Forestry, Beijing 100091, China 2 Research Institute of Forest Ecology, Environment and Protection, Chinese Academy of Forestry, Beijing 100091, China * Correspondence: [email protected] Received: 5 June 2019; Accepted: 12 July 2019; Published: 15 July 2019 Abstract: Quercus bawanglingensis Huang, Li et Xing, an endemic evergreen oak of the genus Quercus (Fagaceae) in China, is currently listed in the Red List of Chinese Plants as a vulnerable (VU) plant. No chloroplast (cp) genome information is currently available for Q. bawanglingensis, which would be essential for the establishment of guidelines for its conservation and breeding. In the present study, the cp genome of Q. bawanglingensis was sequenced and assembled into double-stranded circular DNA with a length of 161,394 bp. Two inverted repeats (IRs) with a total of 51,730 bp were identified, and the rest of the sequence was separated into two single-copy regions, namely, a large single-copy (LSC) region (90,628 bp) and a small single-copy (SSC) region (19,036 bp). The genome of Q. bawanglingensis contains 134 genes (86 protein-coding genes, 40 tRNAs and eight rRNAs). More forward (29) than inverted long repeats (21) are distributed in the cp genome. -

The First Chloroplast Genome Sequence of Boswellia Sacra, a Resin-Producing Plant in Oman
RESEARCH ARTICLE The First Chloroplast Genome Sequence of Boswellia sacra, a Resin-Producing Plant in Oman Abdul Latif Khan1, Ahmed Al-Harrasi1*, Sajjad Asaf2, Chang Eon Park2, Gun-Seok Park2, Abdur Rahim Khan2, In-Jung Lee2, Ahmed Al-Rawahi1, Jae-Ho Shin2* 1 UoN Chair of Oman's Medicinal Plants & Marine Natural Products, University of Nizwa, Nizwa, Oman, 2 School of Applied Biosciences, Kyungpook National University, Daegu, Republic of Korea a1111111111 * [email protected] (AAH); [email protected] (JHS) a1111111111 a1111111111 a1111111111 Abstract a1111111111 Boswellia sacra (Burseraceae), a keystone endemic species, is famous for the production of fragrant oleo-gum resin. However, the genetic make-up especially the genomic informa- tion about chloroplast is still unknown. Here, we described for the first time the chloroplast OPEN ACCESS (cp) genome of B. sacra. The complete cp sequence revealed a circular genome of 160,543 Citation: Khan AL, Al-Harrasi A, Asaf S, Park CE, bp size with 37.61% GC content. The cp genome is a typical quadripartite chloroplast struc- Park G-S, Khan AR, et al. (2017) The First ture with inverted repeats (IRs 26,763 bp) separated by small single copy (SSC; 18,962 bp) Chloroplast Genome Sequence of Boswellia sacra, and large single copy (LSC; 88,055 bp) regions. De novo assembly and annotation showed a Resin-Producing Plant in Oman. PLoS ONE 12 the presence of 114 unique genes with 83 protein-coding regions. The phylogenetic analysis (1): e0169794. doi:10.1371/journal.pone.0169794 revealed that the B. sacra cp genome is closely related to the cp genome of Azadirachta Editor: Xiu-Qing Li, Agriculture and Agri-Food indica and Citrus sinensis, while most of the syntenic differences were found in the non-cod- Canada, CANADA ing regions. -

De Novo Transcriptome Analysis of Dalbergia Odorifera T
Article De Novo Transcriptome Analysis of Dalbergia odorifera T. Chen (Fabaceae) and Transferability of SSR Markers Developed from the Transcriptome Fu-Mei Liu 1,2 , Zhou Hong 3,*, Zeng-Jiang Yang 3, Ning-Nan Zhang 3, Xiao-Jin Liu 3 and Da-Ping Xu 3 1 State Key Laboratory of Tree Genetics and Breeding, Northeast Forestry University, Harbin 150040, China; [email protected] 2 The Experimental Centre of Tropical Forestry, Chinese Academy of Forestry, Pingxiang 532600, China 3 Research Institute of Tropical Forestry, Chinese Academy of Forestry, Longdong, Guangzhou 510520, China; [email protected] (Z.-J.Y.); [email protected] (N.-N.Z.); [email protected] (X.-J.L.); [email protected] (D.-P.X.) * Correspondence: [email protected]; Tel.: +86-20-8703-1037 Received: 15 December 2018; Accepted: 24 January 2019; Published: 26 January 2019 Abstract: Dalbergia odorifera T. Chen (Fabaceae), indigenous to Hainan Island, is a precious rosewood (Hainan hualimu) in China. However, only limited genomic information is available which has resulted in a lack of molecular markers, limiting the development and utilization of the germplasm resources. In this study, we aim to enrich genomic information of D. odorifera, and develop a series of transferable simple sequence repeat (SSR) markers for Dalbergia species. Therefore, we performed transcriptome sequencing for D. odorifera by pooling leaf tissues from three trees. A dataset of 138,516,418 reads was identified and assembled into 115,292 unigenes. Moreover, 35,774 simple sequence repeats (SSRs) were identified as potential SSR markers. A set of 19 SSR markers was successfully transferred across species of Dalbergia odorifera T. -

Quercus Drymeja Unger and Q. Mediterranea Unger
Review of Palaeobotany and Palynology 241 (2017) 98–128 Contents lists available at ScienceDirect Review of Palaeobotany and Palynology journal homepage: www.elsevier.com/locate/revpalbo Taxonomy and palaeoecology of two widespread western Eurasian Neogene sclerophyllous oak species: Quercus drymeja Unger and Q. mediterranea Unger Thomas Denk a,⁎, Dimitrios Velitzelos b,TuncayH.Günerc, Johannes M. Bouchal a,d, Friðgeir Grímsson d,GuidoW.Grimmd,e a Swedish Museum of Natural History, Department of Palaeobiology, Box 50007, 10405 Stockholm, Sweden b National and Kapodistrian University of Athens, Faculty of Geology and Geoenvironment, Department of Historical Geology and Paleontology, Panepistimiopolis, Athens 15784, Greece c Istanbul University, Faculty of Forestry, Department of Forest Botany, 34473 Bahceköy, Istanbul, Turkey d University of Vienna, Department of Palaeontology, 1090 Vienna, Austria e Unaffiliated, 45100 Orléans, France article info abstract Article history: Sclerophyllous oaks (genus Quercus) play important roles in Neogene ecosystems of south-western Eurasia. Received 31 May 2016 Modern analogues (‘nearest living relatives’) for these oaks have been sought among five of six infrageneric lin- Accepted 30 January 2017 eages of Quercus, distributed across the entire Northern Hemisphere. A revision of leaf fossils from lower Miocene Available online 10 February 2017 to Pliocene deposits suggests that morphotypes of the Quercus drymeja complex are very similar to a number of extant Himalayan, East Asian, and Southeast Asian species of Quercus Group Ilex and may indicate subtropical, Keywords: Quercus Group Ilex relatively humid conditions. Quercus mediterranea comprises leaf morphotypes that are encountered in modern Plant fossil Mediterranean species of Quercus Group Ilex, but also in Himalayan and East Asian members of this group indi- Modern analogue cating fully humid or summer-wet conditions. -

The Archaeobotany of Khao Sam Kaeo and Phu Khao Thong: the Agriculture of Late Prehistoric Southern Thailand (Volume 1)
The Archaeobotany of Khao Sam Kaeo and Phu Khao Thong: The Agriculture of Late Prehistoric Southern Thailand (Volume 1) Cristina Castillo Institute of Archaeology University College London Thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy of University College London 2013 Declaration I hereby declare that this dissertation consists of original work undertaken by the undersigned. Where other sources of information have been used, they have been acknowledged. Cristina Castillo October 2013 Institute of Archaeology, UCL 2 Abstract The Thai-Malay Peninsula lies at the heart of Southeast Asia. Geographically, the narrowest point is forty kilometres and forms a barrier against straightforward navigation from the Indian Ocean to the South China Sea and vice versa. This would have either led vessels to cabotage the southernmost part of the peninsula or portage across the peninsula to avoid circumnavigating. The peninsula made easy crossing points strategic locations commercially and politically. Early movements of people along exchange routes would have required areas for rest, ports, repair of boats and replenishment of goods. These feeder stations may have grown to become entrepôts and urban centres. This study investigates the archaeobotany of two sites in the Thai-Malay Peninsula, Khao Sam Kaeo and Phu Khao Thong. Khao Sam Kaeo is located on the east whereas Phu Khao Thong lies on the west of the peninsula and both date to the Late Prehistoric period (ca. 400-100 BC). Khao Sam Kaeo has been identified as the earliest urban site from the Late Prehistoric period in Southeast Asia engaged in trans-Asiatic exchange networks. -

82864099.Pdf
ORIGINAL RESEARCH published: 21 February 2017 doi: 10.3389/fpls.2017.00229 Introgression Threatens the Genetic Diversity of Quercus austrocochinchinensis (Fagaceae), an Endangered Oak: A Case Inferred by Molecular Markers Miao An 1, 2, Min Deng 1, 2*, Si-Si Zheng 1, 2, Xiao-Long Jiang 1, 2 and Yi-Gang Song 1, 2 1 Shanghai Key Laboratory of Plant Functional Genomics and Resources, Shanghai Chenshan Botanical Garden, Shanghai, China, 2 Shanghai Chenshan Plant Science Research Center, Chinese Academy of Sciences, Shanghai, China Natural introgression can cause negative effects where rare species experience genetic assimilation and invade by their abundant congeners. Quercus austrocochinchinensis and Q. kerrii (subgenus Cyclobalanopsis) are a pair of closely related species in the Edited by: Indo-China area. Morphological intermediates of the two species have been reported Scott V. Edwards, in this region. In this study, we used AFLP, SSR and two key leaf morphological Harvard University, USA diagnostic traits to study the two Q. austrocochinchinensis populations, two pure Reviewed by: Octavio Salgueiro Paulo, Q. kerrii and two putative hybrid populations in China. Rates of individual admixture Universidade de Lisboa, Portugal were examined using the Bayesian clustering programs STRUCTURE and NewHybrids, Yongpeng Ma, Kunming Institute of Botany (CAS), with no a priori species assignment. In total, we obtained 151 SSR alleles and China 781 polymorphic loci of AFLP markers. Population differentiation inferred by SSR *Correspondence: and AFLP was incoherent with recognized species boundaries. Bayesian admixture Min Deng analyses and principal coordinate analysis identified more hybrids and backcrossed [email protected] individuals than morphological intermediates in the populations. -

FAGACEAE 1. FAGUS Linnaeus, Sp. Pl. 2: 997. 1753
Flora of China 4: 314–400. 1999. 1 FAGACEAE 壳斗科 qiao dou ke Huang Chengjiu (黄成就 Huang Ching-chieu)1, Zhang Yongtian (张永田 Chang Yong-tian)2; Bruce Bartholomew3 Trees or rarely shrubs, monoecions, evergreen or deciduous. Stipules usually early deciduous. Leaves alternate, sometimes false-whorled in Cyclobalanopsis. Inflorescences unisexual or androgynous with female cupules at the base of an otherwise male inflorescence. Male inflorescences a pendulous head or erect or pendulous catkin, sometimes branched; flowers in dense cymules. Male flower: sepals 4–6(–9), scalelike, connate or distinct; petals absent; filaments filiform; anthers dorsifixed or versatile, opening by longitudinal slits; with or without a rudimentary pistil. Female inflorescences of 1–7 or more flowers subtended individually or collectively by a cupule formed from numerous fused bracts, arranged individually or in small groups along an axis or at base of an androgynous inflorescence or on a separate axis. Female flower: perianth 1–7 or more; pistil 1; ovary inferior, 3–6(– 9)-loculed; style and carpels as many as locules; placentation axile; ovules 2 per locule. Fruit a nut. Seed usually solitary by abortion (but may be more than 1 in Castanea, Castanopsis, Fagus, and Formanodendron), without endosperm; embryo large. Seven to 12 genera (depending on interpretation) and 900–1000 species: worldwide except for tropical and S Africa; seven genera and 294 species (163 endemic, at least three introduced) in China. Many species are important timber trees. Nuts of Fagus, Castanea, and of most Castanopsis species are edible, and oil is extracted from nuts of Fagus. Nuts of most species of this family contain copious amounts of water soluble tannin. -
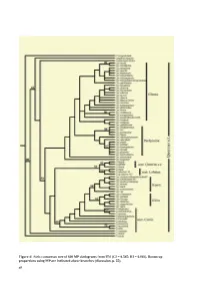
Taxonomy and Systematics of Quercus Subgenus Cyclobalanopsis
Figure 4/ Strict consensus tree of 600 MP cladograms from ITS (CI = 0.545; RI = 0.803). Bootstrap proportions using MP are indicated above branches (discussion, p. 55). 48 Taxonomy and Systematics of Quercus Subgenus Cyclobalanopsis Min Deng1, Zhekun Zhou2*, Qiansheng Li3 2. Xishuangbanna Tropical Botanical Garden, 3. School of Ecology, the Chinese Academy of Sciences, Shanghai Institute of Technology, Kunming, 650223, China. 100 Haiquan Rd., Shanghai, 201418, China 1. Chenshan Plant Sciences Research Center, the Chinese Academy of Sciences, Chenshan Botanical Garden, 3888 ChenHua Rd., Shanghai, 201602, China. Phone: +86-21- 57 79 93 82; Fax: + 86-21-67657811. [email protected]; ABSTRACT Quercus subgenus Cyclobalanopsis is one of the dominant woody plant groups in E and SE Asia, but comprehensive studies on its systematics and taxonomy are limited. In this study, we compared the leaf epidermal and acorn features of 52 species in subgenus Cyclobalanopsis and 15 species from Quercus subgenus Quercus. We also studied molecular phylogeny using DNA sequences from the nuclear ribosomal internal transcribed spacer (ITS) region and the chloroplast psbA–trnH and trnT–trnL regions. Both the leaf epidermal and acorn features indicated ʏve morphologically distinct groups in Cyclobalanopsis: 1) Gilva group (fused stellate trichomes with compound trichome base); 2) Kerrii group (with fasciculate trichomes, and radicles emerging from the basal seed scar; 3) Pachyloma group (papillae on epidermal cells, but glabrous when mature and densely yellowish woolly on the cupule walls); 4) Jenseniana group (lamellae mostly fused to the cupule wall with only the rims free); 5) Glauca group (appressed-lateral-attached trichomes). -

2010-41 Panadda Larpkern (INA).Pdf
Philosophiae Doctor (P <]hYjle]flg^=[gdg_qYf\FYlmjYdJ]kgmj[]EYfY_]e]fl Fgjo]_aYfMfan]jkalqg^Da^]K[a]f[]kMfan]jkal]l]l^gjeadb¬ Woody species regeneration and diversity in a seasonally dry forest in northeastern h D) Thesis 2010:41 Thailand J]cjmll]jaf_g_\an]jkal]l`gklj]Yjl]ja]fl¬jc]mlkYllkcg_af¬j\¬klj] Thailand Panadda Larpkern %g_Zagnal]fkcYh Woodyspeciesregenerationanddiversityinaseasonallydry forestinnortheasternThailand #)0322#0',%-%"'4#01'2#2&-120#02#0'#,20)#321221)-%',0"120#&'*," &'*-1-.&'#-!2-0&'1 ,""0.)#0, #.2T-$!-*-%7," 230*0#1-30!#!,%#+#,2 -05#%',$,'4#01'27-$'$#%!'#,!#1 &1TRSR '1,3+ #0TRSRSVS -%% SWRUVSXXY -%3 [YZVZTVWYWVR[WSVV PhD supervisors: Professor Stein R. Moe Department of Ecology and Natural Resource Management Norwegian University of Life Sciences P.O. Box 5003 NO-1432 Ås, Norway Professor Ørjan Totland Department of Ecology and Natural Resource Management Norwegian University of Life Sciences P.O. Box 5003 NO-1432 Ås, Norway Adjunction committee: Dr. Graciela M. Rusch Norwegian Institute for Nature Research Terrestrisk avd. NO-7485 Trondheim, Norway Professor Jens M. Olesen Department of Biological Sciences Aarhus University DK-8000, Denmark Professor Tron Eid Department of Ecology and Natural Resource Management Norwegian University of Life Sciences P.O. Box 5003 NO-1432 Ås, Norway ii Acknowledgement This thesis is submitted in partial fulfillment of the requirements for the Philosophiae Doctor (PhD) degree at the Norwegian University of Life Sciences (UMB). The PhD scholarship was covered by the quota program of the Norwegian State Educational Loan Fund. I would like also to thank the Department of Ecology and Natural Resource Management (INA) for providing me additional financial support. -

Seasonally Dry Tropical Forests in Continental Southeast Asia Structure, Composition, and Dynamics
1 Seasonally Dry Tropical Forests in Continental Southeast Asia Structure, Composition, and Dynamics Sarayudh Bunyavejchewin, Patrick J. Baker, and Stuart J. Davies he forests of continental Southeast Asia make up a significant portion of the Indo-Burma biodiversity hotspot (see McShea and Davies, this volume). These Tdiverse forests are under severe threat from both land use and climate change, and urgently need to be more sustainably managed. This management of seasonally dry forests in Southeast Asia needs to be based on a sound understanding of the ecol- ogy of natural forests, the habitat requirements of their constituent species, and the response of these systems to natural disturbance dynamics. Natural forest management strategies will help protect habitat for wildlife, will limit the impact of nonnatural dis- turbances, and will lead to opportunities for restoring degraded lands to functional for- ests. An important first step in this process is to develop a much more refined descrip- tion of the forests with a clear understanding of what controls their spatial variation in structure and composition across the region. The vegetation types and ecoregions described in recent mapping and conservation assessments in continental Southeast Asia—for example, Blasco et al. (1996) and Wikramanayake et al. (2002)—are useful for broad-scale threat assessment and priority setting; however, for the active forest management that is required in much of the region now, we need detailed understand- ing of the ecology and dynamics of specific forest types. Our research aims to understand the controls on spatial distributions and temporal dynamics of seasonally dry tropical forest (SDTF) formations across the region.