Study of Fluorescent Pigments in Lepidoptera by Means of Paper Partition Chromatography!
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Papilio Glaucus, P. Marcellus, P. Philenor, Pieris Rapae, Colias Philo Dice, Antho Caris Genutia, Anaea Andria, Euptychia Gemma
102 REMINGTON: 1952 Central Season Vol.7, nos.3·4 Papilio glaucus, P. marcellus, P. philenor, Pieris rapae, Colias philo dice, Antho caris genutia, Anaea andria, Euptychia gemma. One exception to the general scarcity was the large number of Erynnis brizo and E. juvenalis which were seen clustered around damp spots in a dry branch on April 9. MERRITT counted 67 Erynnis and 2 Papilio glaucus around one such spOt and 45 Erynnis around another. Only one specimen of Incisalia henrici was seen this spring. MERRITT was pleased to find Incisalia niphon still present in a small tract of pine although the area was swept by a ground fire in 1951. Vanessa cardui appeared sparingly from June 12 on, the first since 1947. In the late summer the season appeared normal. Eurema lisa, Nathalis iole, Lycaena thoe, and Hylephila phyleus were common. Junonia coenia was more abundant around Louisville than he has ever seen it. A rarity taken in Louisville this fall was Atlides halesus, the first seen since 1948. The latest seasonal record made by Merritt was a specimen of Colias eury theme flying south very fast on December 7. EDWARD WELLING sent a record of finding Lagoa crispata on June 27 at Covington. Contributors: F. R. ARNHOLD; E. G. BAILEY; RALPH BEEBE; S. M. COX; H. V. DALY; 1. W . GRIEWISCH; J. B. HAYES; R. W. HODGES; VONTA P. HYNES; R. LEUSCHNER; J. R. MERRITT; J. H. NEWMAN; M. C. NIEL SEN; 1. S. PHILLIPS; P. S. REMINGTON; WM. SIEKER; EDWARD VOSS; W. H . WAGNER, JR.; E. C. -

Karl Jordan: a Life in Systematics
AN ABSTRACT OF THE DISSERTATION OF Kristin Renee Johnson for the degree of Doctor of Philosophy in History of SciencePresented on July 21, 2003. Title: Karl Jordan: A Life in Systematics Abstract approved: Paul Lawrence Farber Karl Jordan (1861-1959) was an extraordinarily productive entomologist who influenced the development of systematics, entomology, and naturalists' theoretical framework as well as their practice. He has been a figure in existing accounts of the naturalist tradition between 1890 and 1940 that have defended the relative contribution of naturalists to the modem evolutionary synthesis. These accounts, while useful, have primarily examined the natural history of the period in view of how it led to developments in the 193 Os and 40s, removing pre-Synthesis naturalists like Jordan from their research programs, institutional contexts, and disciplinary homes, for the sake of synthesis narratives. This dissertation redresses this picture by examining a naturalist, who, although often cited as important in the synthesis, is more accurately viewed as a man working on the problems of an earlier period. This study examines the specific problems that concerned Jordan, as well as the dynamic institutional, international, theoretical and methodological context of entomology and natural history during his lifetime. It focuses upon how the context in which natural history has been done changed greatly during Jordan's life time, and discusses the role of these changes in both placing naturalists on the defensive among an array of new disciplines and attitudes in science, and providing them with new tools and justifications for doing natural history. One of the primary intents of this study is to demonstrate the many different motives and conditions through which naturalists came to and worked in natural history. -

Lepidoptera: Pieridae) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Sáfián, Sz. Behaviour and development of Pseudopontia gola Sáfián & Mitter, 2011 (Lepidoptera: Pieridae) SHILAP Revista de Lepidopterología, vol. 43, núm. 169, marzo, 2015, pp. 85-89 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45538652010 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 85-89 Behaviour and development 21/3/15 19:32 Página 85 SHILAP Revta. lepid., 43 (169), marzo 2015: 85-89 eISSN: 2340-4078 ISSN: 0300-5267 Behaviour and development of Pseudopontia gola Sáfián & Mitter, 2011 (Lepidoptera: Pieridae) Sz. Sáfián Abstract Information on adult behaviour and development of Pseudopontia gola Sáfián & Mitter, 2011 and foodplant records are presented in this paper, along with a short morphological description of its pre-imaginal stages. Although imagos within the genus Pseudopontia could not be distinguished based on macro-morphological features, there are small but clear morphological differences between the larva and pupa of P. gola and P. zambezi. From the limited number of records, larvae of P. gola seem to utilise a single foodplant species in the Opiliaceae, which differ from, but is related to those of P. paradoxa and P. zambezi. These differences also serve as further evidences of the specific status of P. gola. KEY WORDS: Lepidoptera, Pieridae, Pseudopontia, foodplant, egg, larva, pupa. -

Papilio (New Series) #24 2016 Issn 2372-9449
PAPILIO (NEW SERIES) #24 2016 ISSN 2372-9449 MEAD’S BUTTERFLIES IN COLORADO, 1871 by James A. Scott, Ph.D. in entomology, University of California Berkeley, 1972 (e-mail: [email protected]) Table of Contents Introduction………………………………………………………..……….……………….p. 1 Locations of Localities Mentioned Below…………………………………..……..……….p. 7 Summary of Butterflies Collected at Mead’s Major Localities………………….…..……..p. 8 Mead’s Butterflies, Sorted by Butterfly Species…………………………………………..p. 11 Diary of Mead’s Travels and Butterflies Collected……………………………….……….p. 43 Identity of Mead’s Field Names for Butterflies he Collected……………………….…….p. 64 Discussion and Conclusions………………………………………………….……………p. 66 Acknowledgments………………………………………………………….……………...p. 67 Literature Cited……………………………………………………………….………...….p. 67 Table 1………………………………………………………………………….………..….p. 6 Table 2……………………………………………………………………………………..p. 37 Introduction Theodore L. Mead (1852-1936) visited central Colorado from June to September 1871 to collect butterflies. Considerable effort has been spent trying to determine the identities of the butterflies he collected for his future father-in-law William Henry Edwards, and where he collected them. Brown (1956) tried to deduce his itinerary based on the specimens and the few letters etc. available to him then. Brown (1964-1987) designated lectotypes and neotypes for the names of the butterflies that William Henry Edwards described, including 24 based on Mead’s specimens. Brown & Brown (1996) published many later-discovered letters written by Mead describing his travels and collections. Calhoun (2013) purchased Mead’s journal and published Mead’s brief journal descriptions of his collecting efforts and his travels by stage and horseback and walking, and Calhoun commented on some of the butterflies he collected (especially lectotypes). Calhoun (2015a) published an abbreviated summary of Mead’s travels using those improved locations from the journal etc., and detailed the type localities of some of the butterflies named from Mead specimens. -

Annual Report 2011
Annual Report 2011 © RMCA www.africamuseum.be Foreword 2 Foreword The Royal Museum for Central Africa (RMCA) pub- and culture exhibition was extended, while RMCA lishes a beautiful and richly illustrated annual collection pieces were admired in more than 20 report in book form every two years. In intervening major exhibitions held in different parts of the years – such as 2011 – we publish a digital edition globe. Nearly 30,000 children attended our edu- that is available on our website, and for which a cational workshops or school activities, while our hard copy can be produced on demand. Despite colla boration with African communities became its size, the report is not exhaustive. Rather, it more streamlined. We felt a pang of regret at the seeks to provide the most varied overview pos- departure of ‘our’ elephants in 2011. After grac- sible of our many museum-related, educational, ing our museum’s entrance for three years, the scientific, and other activities on the national and 9 pachyderms that formed the work created by international scene. The long governmental crisis South African artist Andries Botha, You can buy my of 2011 notwithstanding, RMCA was highly pro- heart and my soul, left Tervuren Park for good. ductive and remains one of the most important Africa-focused research institutions, particularly 2011 was also a fruitful year in terms of scientific for Central Africa. research. To highlight the multidisciplinary nature that is the strength of our institution, we organ- As with the previous year, the renovation was one ized ‘Science Days’ for the first time. -

Spatial and Matrix Influences on the Biogeography of Insect Taxa in Forest Fragments in Central Uganda
Spatial and matrix influences on the biogeography of insect taxa in forest fragments in central Uganda Perpetra Akite Dissertation for a cotutelle award of Doctor of Philosophy Degree of Makerere University, Uganda and University of Bergen, Norway Makerere University University of Bergen 2016 Department of Biological Sciences, Makerere University Department of Biology, University of Bergen ii DECLARATION OF ORIGINALITY This is my own work and it has never been submitted for any degree award in any University iii TABLE OF CONTENTS DECLARATION OF ORIGINALITY......................................................................................iii LIST OF CONTENTS...............................................................................................................iv ACKNOWLEDGEMENTS.......................................................................................................vi LIST OF PAPERS....................................................................................................................vii Declaration of authors’ contributions…………………….…...……………...……...viii ABSTRACT...............................................................................................................................x BACKGROUND........................................................................................................................1 Problem statement..........................................................................................................……….2 Objectives........................................................................................................................3 -
Appendix H Terrestrial and Aquatic Species Viability Tables
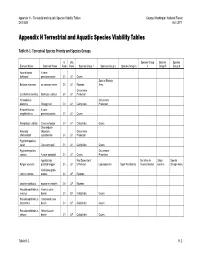
Appendix H – Terrestrial and Aquatic Species Viability Tables George Washington National Forest Draft EIS April 2011 Appendix H Terrestrial and Aquatic Species Viability Tables Table H‐1. Terrestrial Species Priority and Species Groups. G Unit Species Group Species Species Element Name Common Name Rank Rank Species Group 1 Species Group 2 Species Group 3 4 Group 5 Group 6 Apochthonius A cave holsingeri pseudoscorpion G1 U1 Caves Special Biologic Boltonia montana no common name G1 U1 Riparian Area Occurrence Corallorhiza bentleyi Bentley's coalroot G1 U1 Protection Helicodiscus Occurrence diadema Shaggy coil G1 U1 Calciphiles Protection Kleptochthonius A cave anophthalmus pseudoscorpion G1 U1 Caves Nampabius turbator Cave centipede G1 U1 Calciphiles Caves Shenandoah Nannaria Mountain Occurrence shenandoah xystodesmid G1 U1 Protection Pygmarrhopalites sacer Cave springtail G1 U1 Calciphiles Caves Pygmarrhopalites Occurrence caedus A cave springtail G1 U1 Caves Protection Appalachian Fire Dependent/ Sensitive to Shale Special Pyrgus wyandot grizzled skipper G1 U1 Enhanced Lepidopterans Open Woodlands Over-Collection barrens Biologic Area Kankakee globe- Iliamna remota mallow G1 UP Riparian Leuctra monticola montane needlefly G1 UP Riparian Pseudanophthalmus Avernus cave avernus beetle G1 UP Calciphiles Caves Pseudanophthalmus Crossroads cave intersectus beetle G1 UP Calciphiles Caves Pseudanophthalmus Nelson's cave nelsoni beetle G1 UP Calciphiles Caves Table H‐1 H‐1 Appendix H – Terrestrial and Aquatic Species Viability Tables George Washington -

Maine State Legislature
MAINE STATE LEGISLATURE The following document is provided by the LAW AND LEGISLATIVE DIGITAL LIBRARY at the Maine State Law and Legislative Reference Library http://legislature.maine.gov/lawlib Reproduced from scanned originals with text recognition applied (searchable text may contain some errors and/or omissions) l 'j l Public Documents of Maine: BEING THE ANNUAL REPORTS OF THE VARIOUS PuOlic Officers an~ Institutions FOR THE YEAR 188 4. VOLUME II. AUGUSTA: SPRAGUE & SON, PRINTERS TO THE STATE. 188 4. Dormitory and Boarding House. White Hall. Laborat')ry. PR:NC:PAL Bu:Ln:~~Gs OF TH:·: S·rA'1'2 CoLLEGE o>· AGR:CULTURE AN:> '!'HE M:,:cHAn:c ARTS, Oiw:w. ANNUAL REPORTS OF THE Trustees, President, Farm Superintendent aml Treasurer OF THE STATE COLLEGE OF AGRICULTURE AND THE MECHANIC ARTS, Orono, Me., 1883. Published agreeably to a Resolve approved February 25, 187 J. , AUGUSTA: SPRAGUE & SON, PRINTERS TO THE STATE. 18 8 4. ' TRUSTEES' REPORT. To his Excellency, t!te Governor, ancl Executive Council of the State of 1~J,aine: The trustees of the State college respectfully submit their sixteenth annual report, . together with the reports of the president nnd members of the Board of Instruction, and of the trensurer of the college. These several reports will show the present condition of the institution. In response to a recommendation of the trnstees in their report of last year, the Legislature appropriated $2,800 to build and equip shops to afford facilities for the instruction of students in several branches of shop work. The plan outlined in the report referred to has been carried out. -

Smithsonian Miscellaneous Collections
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 116, NUMBER 7 (End of Volume) THE BUTTERFLIES OF VIRGINIA (With 31 Plates) BY AUSTIN H. CLARK AND LEILA F. CLARK Smithsonian Institution DEC 89 «f (PUBUCATION 4050) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION DECEMBER 20, 1951 0EC2 01951 SMITHSONIAN MISCELLANEOUS COLLECTIONS VOL. 116, NO. 7, FRONTISPIECE Butterflies of Virginia (From photograph by Frederick M. Bayer. For explanation, see page 195.) SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 116, NUMBER 7 (End of Volume) THE BUTTERFLIES OF VIRGINIA (With 31 Plates) BY AUSTIN H. CLARK AND LEILA F. CLARK Smithsonian Institution z Mi -.££& /ORG (Publication 4050) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION DECEMBER 20, 1951 Zfyt. Borb QBattimovt (preee BALTIMORE, 1ID., D. 6. A. PREFACE Since 1933 we have devoted practically all our leisure time to an intensive study of the butterflies of Virginia. We have regularly spent our annual leave in the State, stopping at various places from which each day we drove out into the surrounding country. In addition to prolonged visits of 2 weeks or more to various towns and cities, we spent many week ends in particularly interesting localities. We have visited all the 100 counties in the State at least twice, most of them many times, and our personal records are from more than 800 locali- ties. We have paid special attention to the Coastal Plain, particularly the great swamps in Nansemond, Norfolk, and Princess Anne Counties, and to the western mountains. Virginia is so large and so diversified that it would have been im- possible for us, without assistance, to have made more than a super- ficial and unsatisfactory study of the local butterflies. -

Molecular Phylogeny and Systematics of the Pieridae (Lepidoptera: Papilionoidea): Higher Classification and Biogeography
Blackwell Publishing LtdOxford, UKZOJZoological Journal of the Linnean Society0024-4082The Lin- nean Society of London, 2006? 2006 147? 239275 Original Article PHYLOGENY AND SYSTEMATICS OF THE PIERIDAEM. F. BRABY ET AL. Zoological Journal of the Linnean Society, 2006, 147, 239–275. With 8 figures Molecular phylogeny and systematics of the Pieridae (Lepidoptera: Papilionoidea): higher classification and Downloaded from https://academic.oup.com/zoolinnean/article-abstract/147/2/239/2631026 by Harvard Library user on 21 November 2018 biogeography MICHAEL F. BRABY1,2*, ROGER VILA1 and NAOMI E. PIERCE1 1Museum of Comparative Zoology, Harvard University, 26 Oxford St, Cambridge, MA 02138, USA 2School of Botany and Zoology, The Australian National University, Canberra, ACT 0200, Australia Received May 2004; accepted for publication October 2005 The systematic relationships of the butterfly family Pieridae are poorly understood. Much of our current under- standing is based primarily on detailed morphological observations made 50–70 years ago. However, the family and its putative four subfamilies and two tribes, have rarely been subjected to rigorous phylogenetic analysis. Here we present results based on an analysis of molecular characters used to reconstruct the phylogeny of the Pieridae in order to infer higher-level classification above the generic level and patterns of historical biogeography. Our sample contained 90 taxa representing 74 genera and six subgenera, or 89% of all genera recognized in the family. Three complementary approaches were -

Learning About Butterflies.Pdf
2 Learning about Butterflies 3 What Is a Butterfly? 3 Looking at a Butterfly 4 Male or Female Butterfly? Butterfly Conservation 5 Kinds of Butterflies A New Consciousness about Butterflies Gardening ToAttract Butterflies Contents 8 Butterfly Life Cycle 8 Complete Metamorphosis Suggested Projects for Personal Discovery 8 Egg Regional Explorations 10 Caterpillar Species Focus 11 Chrysalis 12 Adult Natural History and Behavioral Themes 12 Parasitoids, Predators, and Diseases Analytical Life History Table 13 Passing the Winter References and Resources 14 Growing Butterflies Butterfly Identification 15 Butterfly Habitats, Ecology, and Behavior Butterfly Manuals 15 Habitat Associations Miscellaneous References on Butterflies 16 Larval-Food Plant Interactions Butterfly Gardening Seasonal Appearance Photography Behavior Plant Identification Sources A ctive Periods Societies and Clubs Basking Collecting Equipment and Supplies Adult Feeding Territoriality Appendices Court.ship, Mating, and Oviposition Dispersal and Migration A. Updated List of New York State Butterflies Sheltering and Roosting B. Larval Food Plants of Some Common New York Butterflies C. Flowers Attractive to Butterflies described and at least skeletal life histories known, there is a need for focused studies of life histories, Learning abozct Bzctterfes distributions, behavior, and ecology. A well-known naturalist from the Butterflies, because of their beauty Saugerties, New York, area, Spider and mystique, are our most visible Barbour, has written a "Nature insects. Their images appear in Walk" column for the Woodstock clothing, jewelry, advertisements, Times for many years. In his 17 May magazines, movies, books, literature, 1979 column he reported fascinating and on television. In warm seasons, details about the falcate orange tip, living butterflies attract our atten- one of the loveliest and most tion as they sip nectar from flowers, mysterious butterflies of the North- lazily fly by, or spiral together east: "The falcate orange tip is an upward in the air. -

Species Diversity Report George Washington National Forest Draft EIS April 2011
Appendix F - Species Diversity Report George Washington National Forest Draft EIS April 2011 U.S. Department of Agriculture Forest Service Southern Region Species Diversity Report George Washington National Forest April 2011 Appendix F - Species Diversity Report George Washington National Forest Draft EIS April 2011 Table of Contents Table of Contents ........................................................................................................................... i 1.0 Introduction ............................................................................................................................ 5 2.0 Species Diversity..................................................................................................................... 5 2.1 Ecosystem Context for Species ............................................................................................ 5 2.2 Identification and Screening of Species ............................................................................... 6 3.0 Threatened and Endangered Species ................................................................................... 7 3.1 Threatened and Endangered Species List ............................................................................ 7 3.2 Threatened and Endangered Species Descriptions and Plan Components .......................... 8 3.2.1 Indiana Bat ........................................................................................................................ 8 3.2.2 Virginia Big-Eared Bat ..................................................................................................