Seventy Eight Insect Species Reared from Fungi in an Ancient, Semi-Natural Beech Woodland in the Chilterns R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topic Paper Chilterns Beechwoods
. O O o . 0 O . 0 . O Shoping growth in Docorum Appendices for Topic Paper for the Chilterns Beechwoods SAC A summary/overview of available evidence BOROUGH Dacorum Local Plan (2020-2038) Emerging Strategy for Growth COUNCIL November 2020 Appendices Natural England reports 5 Chilterns Beechwoods Special Area of Conservation 6 Appendix 1: Citation for Chilterns Beechwoods Special Area of Conservation (SAC) 7 Appendix 2: Chilterns Beechwoods SAC Features Matrix 9 Appendix 3: European Site Conservation Objectives for Chilterns Beechwoods Special Area of Conservation Site Code: UK0012724 11 Appendix 4: Site Improvement Plan for Chilterns Beechwoods SAC, 2015 13 Ashridge Commons and Woods SSSI 27 Appendix 5: Ashridge Commons and Woods SSSI citation 28 Appendix 6: Condition summary from Natural England’s website for Ashridge Commons and Woods SSSI 31 Appendix 7: Condition Assessment from Natural England’s website for Ashridge Commons and Woods SSSI 33 Appendix 8: Operations likely to damage the special interest features at Ashridge Commons and Woods, SSSI, Hertfordshire/Buckinghamshire 38 Appendix 9: Views About Management: A statement of English Nature’s views about the management of Ashridge Commons and Woods Site of Special Scientific Interest (SSSI), 2003 40 Tring Woodlands SSSI 44 Appendix 10: Tring Woodlands SSSI citation 45 Appendix 11: Condition summary from Natural England’s website for Tring Woodlands SSSI 48 Appendix 12: Condition Assessment from Natural England’s website for Tring Woodlands SSSI 51 Appendix 13: Operations likely to damage the special interest features at Tring Woodlands SSSI 53 Appendix 14: Views About Management: A statement of English Nature’s views about the management of Tring Woodlands Site of Special Scientific Interest (SSSI), 2003. -
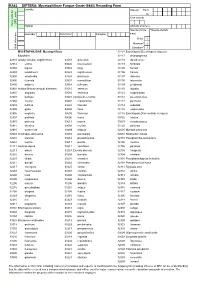
Ra82 Diptera
RA82 DIPTERA: Mycetophilinae Fungus Gnats (6480) Recording Form Locality Date(s) from: to: Vice county GPS users Grey cells for Habitat Altitude (metres) Source (circle *Source details Recorder Determiner Compiler one) Field 1 Museum* 2 Grid reference Literature* 3 MYCETOPHILIDAE: Mycetophilinae 33101 Exechiopsis (Exechiopsis) clypeata Exechiini 33117 dryaspagensis 32801 Allodia (Allodia) anglofennica 33519 griseolum33118 dumitrescae 32912 embla 33526 intermedium33119 fimbriata 32802 lugens 33522 kingi33120 furcata 32803 lundstroemi 33523 nigrofuscum33106 hammi 32804 ornaticollis 33524 proximum33107 indecisa 32806 truncata 33527 rosmellitum33108 intersecta 32805 zaitzevi 33514 ruficorne33109 jenkinsoni 32901 Allodia (Brachycampta) alternans 33515 serenum33110 ligulata 32910 angulata 33516 sericoma33121 magnicauda 32903 barbata 33601 Cordyla brevicornis33112 pseudindecisa 32904 czernyi 33602 crassicornis33113 pulchella 32909 foliifera 33603 fasciata33114 subulata 32905 grata 33604 fissa33115 unguiculata 32906 neglecta 33605 flaviceps33116 Exechiopsis (Xenexechia) crucigera 32907 pistillata 33606 fusca33002 leptura 32915 protenta 33613 insons33003 membranacea 32914 silvatica 33608 murina33122 pollicata 32916 westerholti 33609 nitidula32605 Myrosia maculosa 32602 Allodiopsis domestica 33610 parvipalpis32601 Notolopha cristata 32610 korolevi 33614 pseudomurina32701 Pseudexechia aurivernica 32607 rustica 33611 pusilla32706 monica 32212 Anatella alpina 33612 semiflava32705 parallela 32213 ankeli 33201 Exechia bicincta32703 trisignata 32216 bremia -

Sphaeroceridae (Diptera) in Burrows of Rabbit and Fox in Central Bohemia (Czech Republic), with Description of a New Species of Minilimosina Roháèek
© Entomologica Fennica. 10 September 2019 Sphaeroceridae (Diptera) in burrows of rabbit and fox in central Bohemia (Czech Republic), with description of a new species of Minilimosina Roháèek Jindøich Roháèek Roháèek, J. 2019: Sphaeroceridae (Diptera) in burrows ofrabbit and foxin cen - tral Bohemia (Czech Republic), with description ofa new species of Minilimo- sina Roháèek. Entomol. Fennica 30: 97113. https://doi.org/10.33338/ ef.84085 The communities ofSphaeroceridae in burrows ofEuropean Rabbit Oryctolagus cuniculus and Red Fox Vulpes vulpes in central Bohemia (the Czech Republic) are described including number, dominance and constancy ofspecies and com - pared by means ofa similarity index. A total of17 species were recorded from burrows ofrabbit and 9 fromthose offox. Spelobia talparum (Richards, 1927) and S. pseudonivalis (Dahl, 1909) are considered pholeobiont (= eucoenic) and Spelobia czizeki (Duda, 1918) pholeophilous to pholeobiont species in this habi- tat. Comparison ofthese two communities with those recorded fromother mam- mal subterraneous habitats in Europe revealed that most similar are those from the same locality irrespective ofthe host mammal species or the size ofthe bur- row. The species spectrum ofEuropean Sphaeroceridae recorded from mammal burrows is reviewed and discussed. Minilimosina (Minilimosina) speluncana sp. n. is described on males found in rabbit burrow and its relationship and habitat as- sociation are discussed. J. Roháèek, Silesian Museum, Nádraní okruh 31, CZ-746 01 Opava, Czech Re- public. E-mail: [email protected] Received 3 April 2018, accepted 28 June 2018 1. Introduction undoubtedly inhabited by a rich dipterous com- munity, there are very few reliable data because While the communities offlies(Diptera), includ - most, particularly older, studies were mainly de- ing regularly representatives ofthe family voted to beetles (Coleoptera) and the dipterous Sphaeroceridae, have previously been rather of- component was partly or wholly neglected. -

Recent Noteworthy Findings of Fungus Gnats from Finland and Northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae)
Biodiversity Data Journal 2: e1068 doi: 10.3897/BDJ.2.e1068 Taxonomic paper Recent noteworthy findings of fungus gnats from Finland and northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae) Jevgeni Jakovlev†, Jukka Salmela ‡,§, Alexei Polevoi|, Jouni Penttinen ¶, Noora-Annukka Vartija# † Finnish Environment Insitutute, Helsinki, Finland ‡ Metsähallitus (Natural Heritage Services), Rovaniemi, Finland § Zoological Museum, University of Turku, Turku, Finland | Forest Research Institute KarRC RAS, Petrozavodsk, Russia ¶ Metsähallitus (Natural Heritage Services), Jyväskylä, Finland # Toivakka, Myllyntie, Finland Corresponding author: Jukka Salmela ([email protected]) Academic editor: Vladimir Blagoderov Received: 10 Feb 2014 | Accepted: 01 Apr 2014 | Published: 02 Apr 2014 Citation: Jakovlev J, Salmela J, Polevoi A, Penttinen J, Vartija N (2014) Recent noteworthy findings of fungus gnats from Finland and northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae). Biodiversity Data Journal 2: e1068. doi: 10.3897/BDJ.2.e1068 Abstract New faunistic data on fungus gnats (Diptera: Sciaroidea excluding Sciaridae) from Finland and NW Russia (Karelia and Murmansk Region) are presented. A total of 64 and 34 species are reported for the first time form Finland and Russian Karelia, respectively. Nine of the species are also new for the European fauna: Mycomya shewelli Väisänen, 1984,M. thula Väisänen, 1984, Acnemia trifida Zaitzev, 1982, Coelosia gracilis Johannsen, 1912, Orfelia krivosheinae Zaitzev, 1994, Mycetophila biformis Maximova, 2002, M. monstera Maximova, 2002, M. uschaica Subbotina & Maximova, 2011 and Trichonta palustris Maximova, 2002. Keywords Sciaroidea, Fennoscandia, faunistics © Jakovlev J et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

Diptera: Sphaeroceridae: Limosininae), an Almost Entirely
A review of the Archiceroptera Papp genus complex (Diptera: Sphaeroceridae: Limosininae) by Steven Mark Paiero A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Doctor of Philosophy in Environmental Sciences Guelph, Ontario, Canada © Steven Mark Paiero, December, 2017 ABSTRACT: A review of the Archiceroptera Papp genus complex (Diptera: Sphaeroceridae: Limosininae) Steven Mark Paiero Advisor: University of Guelph, 2017 Dr. S.A. Marshall This thesis has two parts. The first part investigates the relationships between the Archiceroptera genus complex and other members of the Limosininae (Diptera: Sphaeroceridae). A focus is placed on the relationships within the larger epandrial process group, which contains Bitheca, Bromeloecia, Pterogramma, Aptilotella, and Robustagramma, along with Archiceroptera, Rudolfina and several previously unplaced species groups. Molecular and morphological data sets provide the first phylogeny of the group, and were used to support the inclusion of several unplaced species groups within Rudolfina and Archiceroptera, while one new genus is described. Pectinosina gen. nov. includes two species: P. prominens (Duda), previously placed in Rudolfina, and P. carro n. sp. The second part of the thesis deals with revisions of Archiceroptera Papp and Rudolfina Roháček. Rudolfina now includes 13 described species, nine of which are newly described here (R. bucki, R. exuberata, R. howdeni, R. megepandria, R. pauca, R. pilosa, R. newtoni, R. remiforma, and R. tumida). Archiceroptera now includes 29 species, of which 27 are newly described here (A. adamas, A. addenda, A. barberi, A. basilia, A. bilobata, A. bisetosus, A. braziliensis, A. brevivilla, A. browni, A. caliga, A. calligraphia, A. cobolorum, A. -

Zootaxa, the Fungus Gnats (Diptera: Bolitophilidae
Zootaxa 2318: 450–506 (2009) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2009 · Magnolia Press ISSN 1175-5334 (online edition) The fungus gnats (Diptera: Bolitophilidae, Keroplatidae, Mycetophilidae) of Sardinia, with description of six new species* PETER J. CHANDLER 606B Berryfield Lane, Melksham, Wilts SN12 6EL, United Kingdom. E-mail: [email protected] *In: Cerretti, P., Mason, F., Minelli, A., Nardi, G. & Whitmore, D. (Eds), Research on the Terrestrial Arthropods of Sardinia (Italy). Zootaxa, 2318, 1–602. Table of contents Abstract . .450 Introduction . .451 Study area . .452 Material and methods . .452 Abbreviations . .453 Sampling sites . .454 Faunistic list . .456 Bolitophilidae . .456 Keroplatidae, Keroplatinae, Keroplatini . .456 Orfeliini . .457 Macrocerinae . .462 Mycetophilidae, Gnoristinae . .465 Leiinae . .469 Mycetophilinae, Exechiini . .472 Mycetophilini . .480 Mycomyinae . .492 Sciophilinae . .495 Discussion . .500 Acknowledgements . .501 References . .502 Abstract The fungus gnat fauna of Sardinia is reviewed and data presented for all species recorded. Altogether one species of Bolitophilidae, 16 species of Keroplatidae and 105 species of Mycetophilidae are recognised as occurring in Sardinia. As the bolitophilid and two of the mycetophilid species are represented only by females and are not determined to species level, the total confirmed Sardinian list stands at 119 species. Four species of Keroplatidae and 19 species of Mycetophilidae are new to the total Italian fauna, whereas three species of Keroplatidae and 32 species of Mycetophilidae are newly recorded for the island of Sardinia. Six species are described as new to science: two Keroplatidae (Urytalpa juliae sp. nov., Macrocera nuragica sp. nov.) and four Mycetophilidae (Boletina ichnusa sp. nov., Trichonta sandalyon sp. -

Somerset's Ecological Network
Somerset’s Ecological Network Mapping the components of the ecological network in Somerset 2015 Report This report was produced by Michele Bowe, Eleanor Higginson, Jake Chant and Michelle Osbourn of Somerset Wildlife Trust, and Larry Burrows of Somerset County Council, with the support of Dr Kevin Watts of Forest Research. The BEETLE least-cost network model used to produce Somerset’s Ecological Network was developed by Forest Research (Watts et al, 2010). GIS data and mapping was produced with the support of Somerset Environmental Records Centre and First Ecology Somerset Wildlife Trust 34 Wellington Road Taunton TA1 5AW 01823 652 400 Email: [email protected] somersetwildlife.org Front Cover: Broadleaved woodland ecological network in East Mendip Contents 1. Introduction .................................................................................................................... 1 2. Policy and Legislative Background to Ecological Networks ............................................ 3 Introduction ............................................................................................................... 3 Government White Paper on the Natural Environment .............................................. 3 National Planning Policy Framework ......................................................................... 3 The Habitats and Birds Directives ............................................................................. 4 The Conservation of Habitats and Species Regulations 2010 .................................. -

Diptera; Diadocidiidae and Mycetophilidae)
Fungus gnats from Jostedalen, West Norway (Diptera; Diadocidiidae and Mycetophilidae) GEIR E. E. SBLI Snli, G. E. E. 1994. Fungus gnats from Jostedalen, West Norway (Diptera: Diadocidi- idae and Mycetophilidae). Fauna norv. Ser. B 41: 1-12. During a study of terrestrial invertebrates in Jostedalen in 1988, more than 3.000 specimens of fungus gnats were caught. 214 species were recognized, belonging to the families Diadocidiidae and Mycetophilidae. The number of species in Jostedalen is exceptionally high when compared to number of species recorded from other local areas in Europe. The genus Drepanocercus (Vockeroth, 1980) is recorded for the first time from the Palaearctic region. Other rare species are Mycomya simulans Vaisanen, 1984, Acnemia falcata Zaitzev, 1982, Zygom.via pseudohumeralis Caspers, 1980, Anatella aquila Zaitsev, 1989. A. fungina Plassmann, 1984, Exechia subfrigida Las- tovka & Matila, 1974. Exechiopsis dryaspagensis Chandler. 1977 and E. pseudopul- chella (Lundstrom, 1909). Twenty species could not be identified, half of which undoubtly represent undescribed species. The fauna of Norwegian fungus gnats is poorly documented, and most species recorded here are new to Norway. According to the present knowledge on the distribution of fungus gnats, the fauna in Jostedalen seems to have an affinity to the central/eastern Palaearctic fauna, and has more species in common with the Finnish fauna than with the British. Geir E. E. Snli, Museum of Zoology, University of Bergen, Musiplass 3, N-5007 Bergen, Norway. INTRODUCTION The river Jostedola has its origin in the gla- dae, Sciardae and Mycetophilidae. Fungus cier Jostedalsbreen, the largest ice cap on the gnats are distributed all over the world, but European mainland, and runs through the their taxonomy, biology and biogeography valley Jostedalen. -

Bioluminescence in Insect
Int.J.Curr.Microbiol.App.Sci (2018) 7(3): 187-193 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 7 Number 03 (2018) Journal homepage: http://www.ijcmas.com Review Article https://doi.org/10.20546/ijcmas.2018.703.022 Bioluminescence in Insect I. Yimjenjang Longkumer and Ram Kumar* Department of Entomology, Dr. Rajendra Prasad Central Agricultural University, Pusa, Bihar-848125, India *Corresponding author ABSTRACT Bioluminescence is defined as the emission of light from a living organism K e yw or ds that performs some biological function. Bioluminescence is one of the Fireflies, oldest fields of scientific study almost dating from the first written records Bioluminescence , of the ancient Greeks. This article describes the investigations of insect Luciferin luminescence and the crucial role imparted in the activities of insect. Many Article Info facets of this field are easily accessible for investigation without need for Accepted: advanced technology and so, within the History of Science, investigations 04 February 2018 of bioluminescence played a significant role in the establishment of the Available Online: scientific method, and also were among the many visual phenomena to be 10 March 2018 accounted for in developing a theory of light. Introduction Bioluminescence (BL) serves various purposes, including sexual attraction and When a living organism produces and emits courtship, predation and defense (Hastings and light as a result of a chemical reaction, the Wilson, 1976). This process is suggested to process is known as Bioluminescence - bio have arisen after O2 appearance on Earth at means 'living' in Greek while `lumen means least 30 different times during evolution, as 'light' in Latin. -

Insects and Related Arthropods Associated with of Agriculture
USDA United States Department Insects and Related Arthropods Associated with of Agriculture Forest Service Greenleaf Manzanita in Montane Chaparral Pacific Southwest Communities of Northeastern California Research Station General Technical Report Michael A. Valenti George T. Ferrell Alan A. Berryman PSW-GTR- 167 Publisher: Pacific Southwest Research Station Albany, California Forest Service Mailing address: U.S. Department of Agriculture PO Box 245, Berkeley CA 9470 1 -0245 Abstract Valenti, Michael A.; Ferrell, George T.; Berryman, Alan A. 1997. Insects and related arthropods associated with greenleaf manzanita in montane chaparral communities of northeastern California. Gen. Tech. Rep. PSW-GTR-167. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Dept. Agriculture; 26 p. September 1997 Specimens representing 19 orders and 169 arthropod families (mostly insects) were collected from greenleaf manzanita brushfields in northeastern California and identified to species whenever possible. More than500 taxa below the family level wereinventoried, and each listing includes relative frequency of encounter, life stages collected, and dominant role in the greenleaf manzanita community. Specific host relationships are included for some predators and parasitoids. Herbivores, predators, and parasitoids comprised the majority (80 percent) of identified insects and related taxa. Retrieval Terms: Arctostaphylos patula, arthropods, California, insects, manzanita The Authors Michael A. Valenti is Forest Health Specialist, Delaware Department of Agriculture, 2320 S. DuPont Hwy, Dover, DE 19901-5515. George T. Ferrell is a retired Research Entomologist, Pacific Southwest Research Station, 2400 Washington Ave., Redding, CA 96001. Alan A. Berryman is Professor of Entomology, Washington State University, Pullman, WA 99164-6382. All photographs were taken by Michael A. Valenti, except for Figure 2, which was taken by Amy H. -

Hoverflies Family: Syrphidae
Birmingham & Black Country SPECIES ATLAS SERIES Hoverflies Family: Syrphidae Andy Slater Produced by EcoRecord Introduction Hoverflies are members of the Syrphidae family in the very large insect order Diptera ('true flies'). There are around 283 species of hoverfly found in the British Isles, and 176 of these have been recorded in Birmingham and the Black Country. This atlas contains tetrad maps of all of the species recorded in our area based on records held on the EcoRecord database. The records cover the period up to the end of 2019. Myathropa florea Cover image: Chrysotoxum festivum All illustrations and photos by Andy Slater All maps contain Contains Ordnance Survey data © Crown Copyright and database right 2020 Hoverflies Hoverflies are amongst the most colourful and charismatic insects that you might spot in your garden. They truly can be considered the gardener’s fiend as not only are they important pollinators but the larva of many species also help to control aphids! Great places to spot hoverflies are in flowery meadows on flowers such as knapweed, buttercup, hogweed or yarrow or in gardens on plants such as Canadian goldenrod, hebe or buddleia. Quite a few species are instantly recognisable while the appearance of some other species might make you doubt that it is even a hoverfly… Mimicry Many hoverfly species are excellent mimics of bees and wasps, imitating not only their colouring, but also often their shape and behaviour. Sometimes they do this to fool the bees and wasps so they can enter their nests to lay their eggs. Most species however are probably trying to fool potential predators into thinking that they are a hazardous species with a sting or foul taste, even though they are in fact harmless and perfectly edible. -

Hoverfly (Diptera: Syrphidae) Richness and Abundance Vary with Forest Stand Heterogeneity: Preliminary Evidence from a Montane Beech Fir Forest
Eur. J. Entomol. 112(4): 755–769, 2015 doi: 10.14411/eje.2015.083 ISSN 1210-5759 (print), 1802-8829 (online) Hoverfly (Diptera: Syrphidae) richness and abundance vary with forest stand heterogeneity: Preliminary evidence from a montane beech fir forest LAURENT LARRIEU 1, 2, ALAIN CABANETTES 1 and JEAN-PIERRE SARTHOU 3, 4 1 INRA, UMR1201 DYNAFOR, Chemin de Borde Rouge, Auzeville Tolosane, CS 52627, F-31326 Castanet Tolosan Cedex, France; e-mails: [email protected]; [email protected] ² CNPF/ IDF, Antenne de Toulouse, 7 chemin de la Lacade, F-31320 Auzeville Tolosane, France 3 INRA, UMR 1248 AGIR, Chemin de Borde Rouge, Auzeville Tolosane, CS 52627, F-31326 Castanet Tolosan Cedex, France; e-mail: [email protected] 4 University of Toulouse, INP-ENSAT, Avenue de l’Agrobiopôle, F-31326 Castanet Tolosan, France Key words. Diptera, Syrphidae, Abies alba, deadwood, Fagus silvatica, functional diversity, tree-microhabitats, stand heterogeneity Abstract. Hoverflies (Diptera: Syrphidae) provide crucial ecological services and are increasingly used as bioindicators in environ- mental assessment studies. Information is available for a wide range of life history traits at the species level for most Syrphidae but little is recorded about the environmental requirements of forest hoverflies at the stand scale. The aim of this study was to explore whether the structural heterogeneity of a stand influences species richness or abundance of hoverflies in a montane beech-fir forest. We used the catches of Malaise traps set in 2004 and 2007 in three stands in the French Pyrenees, selected to represent a wide range of structural heterogeneity in terms of their vertical structure, tree diversity, deadwood and tree-microhabitats.