Volume 62, Issue 1, 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Julius-Kühn-Archiv Everyone Interested
ICP-PR Honey Bee Protection Group 1980 - 2017 The ICP-PR Bee Protection Group held its rst meeting in Wageningen in 1980 and over the subsequent 38 years it has become the established expert forum for discussing the risks of pesticides to bees and developing solutions how to assess and manage this risk. In recent years, the Bee Protection Group has enlarged its scope of interest from honey bees to many other pollinating insects, such as wild bees including bumble bees. 462 The group organizes international scienti c symposia, usually once in every three years. These are open to Julius-Kühn-Archiv everyone interested. The group tries to involve as many countries as possible, by organizing symposia each time in another European country. It operates with working groups studying speci c problems and propo- Pieter A. Oomen, Jens Pistorius (Editors) sing solutions that are subsequently discussed in plenary symposia. A wide range of international experts active in this eld drawn from regulatory authorities, industry, universities and research institutes participate in the discussions. Hazards of pesticides to bees In the past decade the symposium has largely extended beyond Europe, and is established as the internatio- nal expert forum with participants from several continents. 13th International Symposium of the ICP-PR Bee Protection Group 18. - 20. October 2017, València (Spain) - Proceedings - International Symposium of the ICP-PR Bee Group Protection of the ICP-PR Symposium International th Hazards of pesticides to bees of pesticides - 13 to Hazards 462 2018 Julius Kühn-Institut Bundesforschungsinstitut für Kulturp anzen Julius Kühn-Institut, Bundesforschungsinstitut für Kulturpflanzen (JKI) Veröffentlichungen des JKI Das Julius Kühn-Institut ist eine Bundesoberbehörde und ein Bundesforschungsinstitut. -
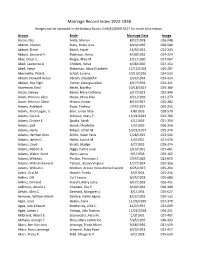
Marriage Record Index 1922-1938 Images Can Be Accessed in the Indiana Room
Marriage Record Index 1922-1938 Images can be accessed in the Indiana Room. Call (812)949-3527 for more information. Groom Bride Marriage Date Image Aaron, Elza Antle, Marion 8/12/1928 026-048 Abbott, Charles Ruby, Hallie June 8/19/1935 030-580 Abbott, Elmer Beach, Hazel 12/9/1922 022-243 Abbott, Leonard H. Robinson, Berta 4/30/1926 024-324 Abel, Oscar C. Ringle, Alice M. 1/11/1930 027-067 Abell, Lawrence A. Childers, Velva 4/28/1930 027-154 Abell, Steve Blakeman, Mary Elizabeth 12/12/1928 026-207 Abernathy, Pete B. Scholl, Lorena 10/15/1926 024-533 Abram, Howard Henry Abram, Elizabeth F. 3/24/1934 029-414 Absher, Roy Elgin Turner, Georgia Lillian 4/17/1926 024-311 Ackerman, Emil Becht, Martha 10/18/1927 025-380 Acton, Dewey Baker, Mary Cathrine 3/17/1923 022-340 Adam, Herman Glen Harpe, Mary Allia 4/11/1936 031-273 Adam, Herman Glenn Hinton, Esther 8/13/1927 025-282 Adams, Adelbert Pope, Thelma 7/14/1927 025-255 Adams, Ancil Logan, Jr. Eiler, Lillian Mae 4/8/1933 028-570 Adams, Cecil A. Johnson, Mary E. 12/21/1923 022-706 Adams, Crozier E. Sparks, Sarah 4/1/1936 031-250 Adams, Earl Snook, Charlotte 1/5/1935 030-250 Adams, Harry Meyer, Lillian M. 10/21/1927 025-376 Adams, Herman Glen Smith, Hazel Irene 2/28/1925 023-502 Adams, James O. Hallet, Louise M. 4/3/1931 027-476 Adams, Lloyd Kirsch, Madge 6/7/1932 028-274 Adams, Robert A. -

Law Enforcement Agency Directory
Michigan Law Enforcement Agencies 08/16/2021 ADRIAN POLICE DEPARTMENT ALBION DPS ALLEGAN POLICE DEPARTMENT CHIEF VINCENT P EMRICK CHIEF SCOTT KIPP CHIEF JAY GIBSON 155 E. MAUMEE STREET 112 W CASS ST 170 MONROE ST ADRIAN MI 49221 ALBION MI 49224 ALLEGAN MI 49010 TX: 517-264-4846 TX: 517-629-3933 TX: 269-673-2115 FAX: 517-264-1927 FAX: 517-629-7828 FAX: 269-673-5170 ADRIAN TOWNSHIP POLICE ALCONA COUNTY SHERIFFS OFFICE ALLEN PARK POLICE DEPARTMENT SHERIFF SCOTT A. STEPHENSON DEPARTMENT CHIEF GARY HANSELMAN 214 WEST MAIN CHIEF CHRISTOPHER S EGAN 2907 TIPTON HWY HARRISVILLE MI 48740 15915 SOUTHFIELD RD ADRIAN MI 49221 TX: 989-724-6271 ALLEN PARK MI 48101 TX: 517-264-1000 FAX: 989-724-6181 TX: 313-386-7800 FAX: 517-265-6300 FAX: 313-386-4158 ALGER COUNTY SHERIFFS OFFICE ADRIAN-BLISSFIELD RAILROAD SHERIFF TODD BROCK ALLEN PARK POLICE POLICE 101 EAST VARNUM STE B DEPARTMENT CHIEF MARK W. DOBRONSKI MUNISING MI 49862 CHIEF CHRISTOPHER S EGAN 38235 N. EXECUTIVE DR. TX: 906-387-4444 15915 SOUTHFIELD RD WESTLAND MI 48185 FAX: 906-387-1728 ALLEN PARK MI 48101 TX: 734-641-2300 TX: 313-386-7800 FAX: 734-641-2323 ALLEGAN COUNTY SHERIFFS FAX: OFFICE AKRON POLICE DEPARTMENT SHERIFF FRANK BAKER ALMA DEPARTMENT OF PUBLIC CHIEF MATTHEW SIMERSON 640 RIVER ST. SAFETY 4380 BEACH STREET ALLEGAN MI 49010 CHIEF KENDRA OVERLA P.O. BOX 295 TX: 269-673-0500 525 EAST SUPERIOR AKRON MI 48701 FAX: 269-673-0406 ALMA MI 48801 TX: 989-691-5354 TX: 989-463-8317 FAX: 989-691-5423 FAX: 989-463-6233 Michigan Law Enforcement Agencies 08/16/2021 ALMONT POLICE DEPARTMENT AMTRAK RAILROAD POLICE ARGENTINE TOWNSHIP POLICE CHIEF ANDREW MARTIN DEPUTY CHIEF JOSEPH DEPARTMENT 817 NORTH MAIN PATTERSON CHIEF DANIEL K ALLEN ALMONT MI 48003 600 DEY ST. -

2012 Annual Report
The year kicked off with a long-awaited Indiana specialty license plate and concluded with a With the groundbreaking ceremony of the International Orangutan record-breaking Christmas celebration. In between, animal conservation was at the forefront in Center came a game-changing moment – this time for orangutans here many ways and included the opening of the outstanding exotic bird exhibit Flights of Fancy, and in the wild. The Center is a unique facility specifically designed to noteworthy births of an elephant and dolphin, and arrivals of a rescued sea lion and baby meet the physical, social, and intellectual needs of these endangered INDIANAPOLIS ZOO walrus. The traditional crowd-pleasing seasonal celebrations also filled the year. And don’t great apes. Its centerpiece, a 150-foot beacon that will be illuminated Annual Report 2012 forget White River Gardens, where orchids flourished in a salute to the natural world. by lights the orangutans turn on, represents the hope that the species not only will survive but also thrive in a world-class environment. Changing the game FOR an ge endangered The Indianapolis Prize Gala showcased our passion for preservation, as this fourth biennial award lauded a distinguished polar bear researcher. The honor was so prestigious it was called the Nobel Prize for the animal conservation world. neration Your generous support is why we celebrate another successful, transformative year. Look inside as we remember some of the highlights and anticipate the challenges ahead. Was 2012 the best year ever for your Indianapolis Zoo? It certainly felt like it. Making a difference for natural world natural for Making adifference THE Dear Friends: We often hear experts speak of the “transformative” power of various entities or ideas. -

Annual Report 2005
2416/D/Rev.1 International Atomic Energy Agency Coordinated Research Activities Annual Report and Statistics for 2007 July 2008 Research Contracts Administration Section Department of Nuclear Sciences and Applications International Atomic Energy Agency http://cra.iaea.org/ TABLE OF CONTENTS EXECUTIVE SUMMARY..................................................................................................................... ii 1. INTRODUCTION .........................................................................................................................1 2. COORDINATED RESEARCH ACTIVITIES IN SUPPORT OF IAEA PROGRAMMES AND SUBPROGRAMMES ..........................................................................................................2 3. COORDINATED RESEARCH ACTIVITIES IN 2007................................................................3 3.1. Member State Participation ..........................................................................................11 3.2. Extra Budgetary Funding..............................................................................................12 3.3. Coordinated Research Projects Completed in 2007 .....................................................12 4. CRP EVALUATION REPORTS FOR COMPLETED CRPS ....................................................13 ANNEX I Total Number of Proposals Received and Awards Made in 2007 ANNEX II Distribution of Total 2007 Contract Awards by Country and Programme ANNEX III Research Coordination Meetings Held in 2007 by Subprogramme ANNEX IV Countries -

April 30, 2016 | Michigan Stadium SPRING COMMENCEMENT UNIVERSITY of MICHIGAN April 30, 2016 10:00 A.M
April 30, 2016 | Michigan Stadium SPRING COMMENCEMENT UNIVERSITY OF MICHIGAN April 30, 2016 10:00 a.m. This program includes a list of the candidates for degrees to be granted upon completion of formal requirements. Candidates for graduate degrees are recommended jointly by the Executive Board of the Horace H. Rackham School of Graduate Studies and the faculty of the school or college awarding the degree. Following the School of Graduate Studies, schools are listed in order of their founding. Candidates within those schools are listed by degree then by specialization, if applicable. Horace H. Rackham School of Graduate Studies ..................................................................................................20 College of Literature, Science, and the Arts ............................................................................................................31 Medical School ......................................................................................................................................................51 Law School ............................................................................................................................................................52 School of Dentistry ................................................................................................................................................54 College of Pharmacy ..............................................................................................................................................55 -

Study of Civilian Personnel in Ordnance Department
PUBLISHED DAZLY under order of THE PRESIDENT of TIZE UNZTXD STATES by COMMITTEE on PUBLIC INFORMATION GEORGE CREEL, Chairman * * COMPLEST Record of U. .. GOVERNMENT Activities LVoL. 2 WASHINGTON, TUESDAY, NOVEMBER 26, 1918. No. 473 STUDY OF CIVILIAN PERSONNEL PASSPORT RULES MODIFIED 14 DU MARU SURVIVORS LAND INORDNANCE DEPARTMENT AS TO DRAFT REGISTRANTS AFTER 37 DAYS INOPEN BOAT Inquiry Being Made to Avoid Un- EXECUTIVE ORDER. Sixieen Die of Hunger and Ex- necessary Hardship When Whereas, by an Executive order under - posure While Drifting 1,200 Reductions Begin. date of August 8, 1918, prescribing rules Miles in the Pacific. The War Department authorizes the and regulations governing departure from The Navy Department is informed that following from the Ordnance Depart- and entry into the United States, it was 14 men of the crew of the steamship ment: provided by section 12 thereof as fol- Du Maru, which was struck by lightning Reduction of the civilian personnel of lows: and sunk on October 16, 20 miles from at San Jose, L'Ori- the Ordnance Department will be carried " No' person registered or enrolled or Guam, have landed out in a way that will Impose the least for mili- ente, P. I., 1,200 miles from Guam, after possible hardship upon civilian employ- subject to registry or enrollment 87 days in an open boat. The men were ees. Maj. Gen. 0. C. Williams, Chief of tary ser'vice in the United States shall without food for the last 10 days of this Ordnance, has jqst made this announce- depart from the United States without the time and for 5 days without water. -

Brain2019 Final Program
TABLE OF CONTENTS Congress Information ISCBFM Welcome Letter .................................................. 2 Committees ...................................................................... 3 About ISCBFM ................................................................. 4 About Yokohama .............................................................. 5 Awards ............................................................................. 6 Information A to Z ............................................................. 8 Information for Chairs & Speakers ..................................12 Access and Floor Guide ..................................................16 Scientific Program ISCBFM Meetings ...........................................................19 Social Events ................................................................. 20 Official Brain & Brain PET 2019 Satellites ...................... 21 Program at a Glance ...................................................... 24 Thursday, July 4th .......................................................... 30 Friday, July 5th ............................................................... 36 Saturday, July 6th ........................................................... 48 Sunday, July 7th ............................................................. 62 Poster Session Friday, July 5th ............................................................... 77 Saturday, July 6th ..........................................................104 Sunday, July 7th ............................................................130 -

Mission 1: Battle Plans
CAMPAIGN 1: SECOND ASSAULT MISSION 1: BATTLE PLANS Door Dark Legion Reinforcement Entrance Point Doomtrooper Entrance Point D Desk D D HIGH COMMAND BRIEFING EVENTS Intelligence reports indicate that the forces The Dark Legion player shuffles event cards of the Dark Legion are amassing for another numbered 1–12 and draws 5 cards. The rest While the others cleansed the The years had been rough on Eva attack. It is imperative that the battle plans for of the event cards are set aside and are not sanctum, Samantha Kempf searched Kempf’s looks, but those eyes! One Alakhai’s next assault are located before the used in the mission. Reinforcements on the the small rooms behind it. She tore could still drown in them. Pretty and plans are finalized. event cards enter the Citadel at entrance down tapestries and kicked things charming Eva. Little sister Eva. Always points in sector tiles 14 & 15. over, longing for something to kill. in the shadow of her brash big sister. MISSION The corpses in the sanctum had been Samantha, her father’s pride. Eva, her The Doomtroopers must enter the DARK LEGION RESOURCES mangled beyond belief. mother’s daughter. Samantha, the Citadel, make their way to the War Room The Dark Legion player removes force cards athlete. Eva, the socialite. Samantha, and get photos of Alakhai’s battle plans numbered 33–36 and sets them aside. The one on the rack had whispered the officer. Eva, the Heretic. Samantha and then upload the plans back to High They are not used in this mission. -

(2011). Covariance Modeling of MRI Brain Volumes in Memory Circuitry in Schizophrenia: Sex Differences Are Critical
1 Table S2. References of studies included in the analyses. Abbs, B., Liang, L., Makris, N., Tsuang, M., Seidman, L. J., & Goldstein, J. M. (2011). Covariance modeling of MRI brain volumes in memory circuitry in schizophrenia: Sex differences are critical. Neuroimage, 56(4), 1865– 1874. doi:10.1016/j.neuroimage.2011.03.079 Acevedo, S. F., Piper, B. J., Craytor, T. S. B., & Raber, J. (2010). Apolipoprotein E4 and sex affect neurobehavioral performance in primary school children. Pediatric Research, 67(3), 293–299. doi:10.1203/PDR.0b013e3181cb8e68 Aikins, D. E., Anticevic, A., Kiehl, K. A., & Krystal, J. H. (2010). Sex-related differences in amygdala activity influences immediate memory. NeuroReport, 21, 273–276. doi:10.1097/WNR.0b013e328335b3f9 Aine, C. J., Adair, J. C., Knoefel, J. E., Hudson, D., Qualls, C., Kovacevic, S., . Stephen, J. M. (2005). Temporal dynamics of age-related differences in auditory incidental verbal learning. Cognitive Brain Research, 24, 1–18. doi:10.1016/j.cogbrainres.2004.10.024 Albus, M., Hubmann, W., Mohr, F., Scherer, J., Sobizack, N., Franz, U., . Wahlheim, C. (1997). Are there gender differences in neuropsychological performance in patients with first-episode schizophrenia? Schizophrenia Research, 28(1), 39–50. Aliotti, N. C. & Rajabiun, D. A. (1991). Visual memory development in preschool children. Perceptual and Motor Skills, 73(3, Pt 1), 792–794. Allwood, C. M., Granhag, P. A., & Jonsson, A.-C. (2006). Child witnesses’ metamemory realism. Scandinavian Journal of Psychology, 47, 461–470. doi:10.1111/j.1467-9459.2006.00530.x Almela, M., van der Meij, L., Hidalgo, V., Villada, C., & Salvador, A. -
Abbinante * Adinolfi * Aiello * Aimola * Albertinelli * Aliprandi * Altobelli * Ambrosino * Ameruoso Belloli * Angelucci *
BUSCAPRONTA www.buscapronta.com ARQUIVO 21 DE PESQUISAS GENEALÓGICAS 194 PÁGINAS – MÉDIA DE 62.500 SOBRENOMES/OCORRÊNCIA Para pesquisar, utilize a ferramenta EDITAR/LOCALIZAR do WORD. A cada vez que você clicar ENTER e aparecer o sobrenome pesquisado GRIFADO (FUNDO PRETO) corresponderá um endereço Internet correspondente que foi pesquisado por nossa equipe. Ao solicitar seus endereços de acesso Internet, informe o SOBRENOME PESQUISADO, o número do ARQUIVO BUSCAPRONTA DIV ou BUSCAPRONTA GEN correspondente e o número de vezes em que encontrou o SOBRENOME PESQUISADO. Número eventualmente existente à direita do sobrenome (e na mesma linha) indica número de pessoas com aquele sobrenome cujas informações genealógicas são apresentadas. O valor de cada endereço Internet solicitado está em nosso site www.buscapronta.com . Para dados especificamente de registros gerais pesquise nos arquivos BUSCAPRONTA DIV. ATENÇÃO: Quando pesquisar em nossos arquivos, ao digitar o sobrenome procurado, faça- o, sempre que julgar necessário, COM E SEM os acentos agudo, grave, circunflexo, crase, til e trema. Sobrenomes com (ç) cedilha, digite também somente com (c) ou com dois esses (ss). Sobrenomes com dois esses (ss), digite com somente um esse (s) e com (ç). (ZZ) digite, também (Z) e vice-versa. (LL) digite, também (L) e vice-versa. Van Wolfgang – pesquise Wolfgang (faça o mesmo com outros complementos: Van der, De la etc) Sobrenomes compostos ( Mendes Caldeira) pesquise separadamente: MENDES e depois CALDEIRA. Tendo dificuldade com caracter Ø HAMMERSHØY – pesquise HAMMERSH HØJBJERG – pesquise JBJERG BUSCAPRONTA não reproduz dados genealógicos das pessoas, sendo necessário acessar os documentos Internet correspondentes para obter tais dados e informações. DESEJAMOS PLENO SUCESSO EM SUA PESQUISA. -
Unclaimed Cash Back Last Names Or Business Names That Start with S
Unclaimed Cash Back Last Names or Business Names that start with S Last Name or Business Name First Name Middle Name Address Amount S & B GEOTHERMAL SAINT FRANCIS Less than $50 S & I INDUSTRIES BLAINE Greater than $50 S & I INDUSTRIES ANOKA Greater than $50 S & J CONTRACTING OF MINNESOTA COON RAPIDS Greater than $50 S & L P BUILDERS INC BROOKLYN PARK Greater than $50 S & S INDUSTRIES II BLAINE Greater than $50 S & T TRANSPORT COON RAPIDS Greater than $50 S & T TRANSPORT COON RAPIDS Greater than $50 S C CABINETS WYOMING Greater than $50 S C I SERVICES OF MPLS MINNETONKA Greater than $50 S J S INC ANOKA Greater than $50 S L P MACHINE INC ANOKA Greater than $50 S R M ENERGY LLC HAMMOND Less than $50 S S INDUSTRIES II INC BLAINE Greater than $50 SAAD MIKE J SAINT PAUL Less than $50 SAAR JESSICA OSSEO Less than $50 SABA DENISE A SAINT PAUL Greater than $50 SABA GERALD J HAM LAKE Greater than $50 SABA FLOWER SHOP ANDOVER Greater than $50 SABBY GERALD LEROY MOUNDSVIEW Greater than $50 SABBY RICHARD L SPRING LAKE PARK Greater than $50 SABELS MARTIN BIG LAKE Less than $50 SABIN TOM W COON RAPIDS Greater than $50 SABLE DEBRA E SAINT PAUL Greater than $50 SABRASKI CURTIS S SAINT PAUL Less than $50 SABRASKI KEVIN JUNO Less than $50 SACCO COLLEEN F BLAINE Less than $50 SACCO MARANDA R CAMBRIDGE Less than $50 SACEVICH JULIE A HUGO Less than $50 SACK STEVE R NEVIS Less than $50 SACKETT EUGENE CEDAR PARK Greater than $50 SACRE KARI L CEDAR Greater than $50 SADERGASKI JOHN LAKE ELMO Less than $50 SADHU AMIT WHITE BEAR LAKE Less than $50 SADLACK ANDREA