Lecture Powerpoint Sense Organs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sound and the Ear Chapter 2
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Chapter© Jones & Bartlett 2 Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Sound and the Ear © Jones Karen &J. Kushla,Bartlett ScD, Learning, CCC-A, FAAA LLC © Jones & Bartlett Learning, LLC Lecturer NOT School FOR of SALE Communication OR DISTRIBUTION Disorders and Deafness NOT FOR SALE OR DISTRIBUTION Kean University © Jones & Bartlett Key Learning, Terms LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR Acceleration DISTRIBUTION Incus NOT FOR SALE OR Saccule DISTRIBUTION Acoustics Inertia Scala media Auditory labyrinth Inner hair cells Scala tympani Basilar membrane Linear scale Scala vestibuli Bel Logarithmic scale Semicircular canals Boyle’s law Malleus Sensorineural hearing loss Broca’s area © Jones & Bartlett Mass Learning, LLC Simple harmonic© Jones motion (SHM) & Bartlett Learning, LLC Brownian motion Membranous labyrinth Sound Cochlea NOT FOR SALE OR Mixed DISTRIBUTION hearing loss Stapedius muscleNOT FOR SALE OR DISTRIBUTION Compression Organ of Corti Stapes Condensation Osseous labyrinth Tectorial membrane Conductive hearing loss Ossicular chain Tensor tympani muscle Decibel (dB) Ossicles Tonotopic organization © Jones Decibel & hearing Bartlett level (dB Learning, HL) LLC Outer ear © Jones Transducer & Bartlett Learning, LLC Decibel sensation level (dB SL) Outer hair cells Traveling wave theory NOT Decibel FOR sound SALE pressure OR level DISTRIBUTION -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Specialized for Sound Detection A. Outer
EAR © 2019zillmusom I. Overview - specialized for sound detection A. Outer ear - funnel shaped structure of cartilage and skin that leads to Tympanic membrane; directs sound toward Tympanic membrane; helps detect source of sound. B. Middle ear - air filled chamber that contains bones (ossicles) that link Tympanic membrane to cochlea; also contains muscles that dampen sounds; middle ear is linked to Nasopharynx by auditory tube which allows for equilibration of air pressure on inner side of Tympanic membrane. C. Inner ear - fluid filled chamber in petrous part of temporal bone; inner ear contains Cochlea (hearing) and Vestibular apparatus for gravity detection (both innervated by CN VIII). Clinical Note: Functioning of inner ear can be tested independently by vibrations transmitted directly through bone (Weber test: tuning fork on calvarium is perceived as sound); CONDUCTIVE HEARING LOSS - damage to middle ear (tympanic membrane, auditory ossicles); SENSORINEURAL HEARING LOSS - damage to inner ear (cochlea, CN VIII). II. Outer Ear - composed of two parts: A. Auricle (pinna) - elastic cartilage covered with skin; functions to reflect sound waves. Parts: helix, antihelix, tragus and lobule. Decorative Note: Cartilage does not extend into Lobule; Lobule can be readily pierced to provide support for decorative metal objects. B. External auditory meatus - tube from auricle to the Tympanic membrane; posterior to Parotid gland and TMJ (Temporomandibular joint); located anterior to mastoid process. Outer third consists of elastic cartilage; contains hairs, sebaceous glands and ceruminous glands (produce cerumen = ear wax); serves to protect Tympanic membrane; Inner two thirds is composed of bone lined with skin. Clinical note: External auditory meatus is curved anteriorly in adults, is straight in children; in adults, auricle is pulled up and back to insert otoscope. -
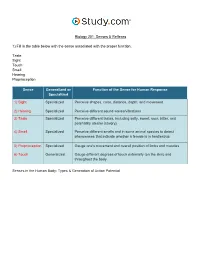
Senses & Reflexes in the Nervous System Visual Worksheet
Biology 201: Senses & Reflexes 1) Fill in the table below with the sense associated with the proper function. Taste Sight Touch Smell Hearing Proprioception Sense Generalized or Function of the Sense for Human Response Specialized 1) Sight Specialized Perceive shapes, color, distance, depth, and movement 2) Hearing Specialized Perceive different sound waves/vibrations 3) Taste Specialized Perceive different tastes, including salty, sweet, sour, bitter, and potentially umami (savory) 4) Smell Specialized Perceive different smells and in some animal species to detect pheromones that indicate whether a female is in heat/estrus 5) Proprioception Specialized Gauge one's movement and overall position of limbs and muscles 6) Touch Generalized Gauge different degrees of touch externally (on the skin) and throughout the body Senses in the Human Body: Types & Generation of Action Potential 2) Label the structures of the eye. Pupil Sclera Retina Lens Iris Cornea The Eye: Structure, Image Detection & Disorders 3) Label the chambers and structures of the eye below. Posterior chamber Muscle Retina Conjunctiva Cornea Sclera Optic nerve Anterior chamber Pupil Choroid layer Blood vessels Lens Iris Vitreous chamber The Eye: Structure, Image Detection & Disorders 4) Label the features of the visual pathway below. Optic chiasm Left cerebral hemisphere Pretectal nucleus Lateral geniculate nucleus of the thalamus Superior colliculus Visual cortex Right cerebral hemisphere The Eye: Structure, Image Detection & Disorders 5) Study the image of the section of the retina below. Label the neural layers. Bipolar cell Cone cell Neural layer Pigmented layer Rod cell Ganglion cell The Eye: Structure, Image Detection & Disorders 6) Label the structures of the nose below. -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Ear Infections in Children
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Ear Infections in Children What is an ear infection? How can I tell if my child has an ear infection? An ear infection is an inflammation of the middle ear, usually caused by bacteria, that occurs when fluid builds Most ear infections happen to children before they’ve up behind the eardrum. Anyone can get an ear infection, learned how to talk. If your child isn’t old enough to say but children get them more often than adults. Five out of “My ear hurts,” here are a few things to look for: six children will have at least one ear infection by their third } Tugging or pulling at the ear(s) birthday. In fact, ear infections are the most common reason parents bring their child to a doctor. The scientific name for } Fussiness and crying an ear infection is otitis media (OM). } Trouble sleeping What are the symptoms of an } Fever (especially in infants and younger children) ear infection? } Fluid draining from the ear } Clumsiness or problems with balance There are three main types of ear infections. Each has a different combination of symptoms. } Trouble hearing or responding to quiet sounds. } Acute otitis media (AOM) is the most common ear What causes an ear infection? infection. Parts of the middle ear are infected and swollen and fluid is trapped behind the eardrum. This An ear infection usually is caused by bacteria and often causes pain in the ear—commonly called an earache. -

KULAK ANATOMİSİ.Pdf
OMÜ SHMYO ANATOMİ KULAK ANATOMİSİ ÖĞR. GÖR. Dr. GÜRSEL AK GÜVEN DİLEK GÜN – 20460810 SELİN ÇETİNKAYA - 20460770 EDANUR ÇEPNİ - 20460780 RÜMEYSA İNAL -20460756 Auris Externa(Dış Kulak) Auris Media (Orta Kulak) Auris İnterna (İç Kulak) Auricula (Kulak Kepçesi) Cavitas Tympani (Timpan Labyrinthus Osseus (Kemik Boşluğu) Labirent) • Ligementa Auricularia • Auris Media’nın Duvarları • Vestibulum • Musculiauriculares • Canales Semicirculares • Auricula’nın Duyu Sinirleri Membrana Tympanica • Cochlea • Auricula’nın Arterleri (Kulak Zarı) • Arterleri Labyrinthus Membranaceus • Auricula’nın Venleri • Venleri (Zar Labirent) • Auricula’nın Lenfatikleri • Sinirleri • Ductus Semicirculares • Utriculus Meatus Acusticus Tuba Auditiva (Östaki • Sacculus Externus (Dış Kulak Yolu) Borusu) • Ductulus Cochlearis • Meatus Acusticus Externus Ossicula Auditus (Kulak • Organum Spirale (Corti Organı) Arterleri Kemikçikleri) • Membrana Tektoria • Meatus Acusticus Externus • Malleus • Incus • Stapes İç Kulak Sıvıları Venleri Musculi Ossiculorum Meatus Acusticus İnternus • Meatus Acusticus Externus Auditorium (Auris (İç Kulak Yolu) Duyu Sinirleri Media’nın Kasları) Auris İnterna’nın Damarları • Meatus Acusticus Externus Auris Media’nın Arterleri • Arterleri • Venleri Lenfatikleri Auris Media’nın Venleri Auris İnterna Sinirleri Auris Media’nın Sinirleri İşitme Siniri ve İşitme Yolları & İşitme Nedir ve Nasıl Gerçekleşir? Kulak Hastalıkları Buşon Hastalığı İşitme ve Sinir Sistemi • Afferent Sistem Timpanik Membran Perforasyonu Hastalığı • Efferent -

Patient Information – Ear Surgery Instructions
The Oregon Clinic, Plaza ENT Division 5050 NE Hoyt #655, Portland, OR 97213 Phone: 5034882400 Fax: 5032310121 Patient Information – Ear Surgery Instructions Pre- and Post-operative Instructions for Ear Surgery (not including ear tubes) Before Surgery: Many ear surgeries involve manipulation of the eardrum (tympanic membrane), and some require the removal of bone to facilitate the treatment of your ear disease. As with any operation, infection, scarring, and blood clot formation (hematoma) are possible. The facial nerve is at risk for injury or temporary weakness during any ear surgery. Dizziness following surgery may be expected. Hearing loss or ringing in the ear (tinnitus) may be more pronounced. Taste disturbance is not uncommon in certain ear surgeries for a few weeks following surgery and, in a few instances, could be prolonged or permanent. An incision may be made behind your ear, on your earlobe, or behind the pointed cartilage in front of your ear (the tragus). These areas normally heal without problems or obvious scars. Hair around the ear may or may not be shaved. Flying is usually permitted one month after surgery. Swimming may be allowed six weeks after surgery, but check with your doctor first before resuming swimming or other water sports. If your work is not strenuous and depending upon the type of surgery you’ve had, you may return to work 3 to 4 days from the date of surgery. Generally, you will be seen about 2-3 weeks after surgery. This gives your eardrum time to heal before we see you back. Pre-operative Instructions: 1. -

Basic Anatomy and Physiology of the Ear 11
1 BASIC ANATOMY AND PHYSIOLOGY OF THE EAR J. Irwin Introduction The ear is a small, complex series of interlinked structures that are involved in both maintenance of normal balance and the sense of hearing. In order to hear, the ear collects the sound waves that arrive as pressure changes in air and converts these into neurochemical impulses that travel along the cochlear- vestibular nerve to the brain. There are both active and passive mechanisms involved in this process.The prime function of the vestibular system is to detect and compensate for movement. This includes the ability to maintain optic fix- ation despite movement and to initiate muscle reflexes to maintain balance. For the purposes of describing structure and function the ear is usually split into four distinct parts. These are the outer ear, the middle ear and the audi- tory and vestibular parts of the inner ear (Figure 1.1). The outer ear This is sometimes known as the external ear and consists of the ear that is visible on the side of the head (the pinna), the external auditory meatus (ear hole) and the ear canal (external auditory canal) that leads to the eardrum (or tympanic membrane). The tympanic membrane has three layers and the outer layer is usually included as part of the outer ear. THE PINNA This is, for the most part, a piece of cartilage covered by skin (Figure 1.2). There is also a fatty earlobe in most people. The skin covering the cartilage is Infection and Hearing Impairment. Edited by V.E. Newton and P.J. -

Effectiveness of Ear Molding in the Treatment of Congenital Auricular Deformity
CHINA CLINIcaL STUDIES IV ORIGINAL ARTICLE Effectiveness Of Ear Molding In the Treatment Of Congenital Auricular Deformity Corresponding authors: CHEN Peiwei 1 LI Jie 2 ZHAO Abstract Objective: To evaluate the short-term efficacy of ear molding in the treatment of congenital Shouqin 1 YANG Jingsong 1 DOU auricular deformity. Jingmin 1 WEI Chenyi 1 Methods: 24 infants (28 ears) were treated with ear molding device (EarWell Infant Ear Correction System). Doctors and parents were surveyed 1 month after treatment. Results: All cases were treated successfully without severe complications. 25 ears (89%) and 26 ears (92%) were rated as very satisfied or satisfied by doctors and parents, respectively. Conclusion: Ear molding is a noninvasive treatment, and effectively corrects congenital auricular deformity. Key words: Ear Disease; Deformity; Retrospective Study; Ear Molding. INFORMATION Exclusion Criteria Auricular deformities are classified into structural malformation and Age over 3 months; premature delivery; weight less than 2.5 kg; morphological malformation [1]. The former usually refers to the hypo- pathological jaundice, pneumonia and other systemic diseases. plasia of the auricle caused by the coloboma of skin and cartilage. The latter is the abnormal morphology of the well-developed auricle, which Overall 24 sick children (28 ears) were recruited for this treatment, could cause negative effects to the psychological development and so- including 14 males, 10 females, 16 right ears and 12 left ears. The age cial activities of children. Unlike structural malformations, such as mi- range for the recruited patients is 17-77 days, with a median age of 40.5 crotia, which must be corrected by auricle reconstruction, the auricle days. -

Intraoperative Neuromonitoring Techniques in the Surgical Management of Acoustic Neuromas
Neurosurg Focus 33 (3):E6, 2012 Intraoperative neuromonitoring techniques in the surgical management of acoustic neuromas TAEMIN OH, B.A.,1 DANIEL T. NAGASAWA, M.D.,1 BRENDAN M. FONG, B.S.,1 ANDY TRANG, B.S.,1 QUINtoN GOPEN, M.D.,3 ANDREW T. PARSA, M.D., PH.D.,2 AND ISAAC YANG, M.D.1,4 Departments of 1Neurosurgery and 3Otolaryngology ENT, David Geffen School of Medicine, University of California, Los Angeles; 4UCLA Jonsson Comprehensive Cancer Center, University of California, Los Angeles; and 2Department of Neurosurgery, UCSF School of Medicine, University of California, San Francisco, California Unfavorable outcomes such as facial paralysis and deafness were once unfortunate probable complications following resection of acoustic neuromas. However, the implementation of intraoperative neuromonitoring during acoustic neuroma surgery has demonstrated placing more emphasis on quality of life and preserving neurological function. A modern review demonstrates a great degree of recent success in this regard. In facial nerve monitoring, the use of modern electromyography along with improvements in microneurosurgery has significantly improved preservation. Recent studies have evaluated the use of video monitoring as an adjunctive tool to further improve outcomes for patients undergoing surgery. Vestibulocochlear nerve monitoring has also been extensively studied, with the most popular techniques including brainstem auditory evoked potential monitoring, electrocochleography, and direct compound nerve action potential monitoring. Among them, direct recording remains the most promising and preferred monitoring method for functional acoustic preservation. However, when compared with postoperative facial nerve function, the hearing preservation is only maintained at a lower rate. Here, the authors analyze the major intraoperative neuromonitoring techniques available for acoustic neuroma resection. -

Reconstruction of Acquired Pinna Defects
Reconstruction of Acquired Pinna Defects Dissertation submitted to THE TAMILNADU Dr. M. G. R. MEDICAL UNIVERSITY In partial fulfillment of the regulations for the award of the degree of MCh (PLASTIC SURGERY) BRANCH III THE TAMIL NADU DR. MGR. MEDICAL UNIVERSITY CHENNAI. AUGUST 2008 DECLARATION I solemnly declare that this dissertation “RECONSTRUCTION OF ACQUIRED PINNA DEFECTS” was prepared by me in the Department of Plastic, Reconstructive and Maxillofacial Surgery, Madras Medical College and Government General Hospital, Chennai under the guidance and supervision of Professor & HOD Department of Plastic, Reconstructive and Maxillofacial Surgery, Madras Medical College and Government General Hospital, Chennai between 2005 and 2008. This dissertation is submitted to the TamilNadu Dr. MGR Medical University, Chennai in partial fulfilment of the University requirements for the award of degree of MCh Plastic Surgery. Place: Chennai Date : CERTIFICATE This is to certify that this dissertation entitled “ RECONSTRUCTION OF ACQUIRED PINNA DEFECTS” is a bonafide record of the research work done by Dr. D.VINOTH KUMAR for the award of MCh Plastic Surgery, under the supervision of Professor & HOD, Plastic Surgery Madras Medical College and Government General Hospital, Chennai between 2005 and 2008. I also certify that this dissertation is the result of the independent work done by candidate. Professor and Head of the Department Dean Plastic, Reconstructive and Maxillofacial Surgery Madras Medical College and Madras Medical College Government General Hospital Chennai – 600 003. Chennai – 600 003. ACKNOWLEDGEMENT I am thankful to the Dean, Madras Medical College and Government General Hospital, Chennai for permitting me to carry out this study. I am very grateful to my teacher and guide Professor V Alamelu M.S., Mch, FICS, FMMS Professor & HOD, Plastic Reconstructive and Maxillofacial Surgery, Madras Medical College, Chennai for her expert guidance and help without which this study would not have been possible.