Neurophysiologic Intraoperative Monitoring of the Vestibulocochlear Nerve
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Cranial Nerves 1, 5, 7-12
Cranial Nerve I Olfactory Nerve Nerve fiber modality: Special sensory afferent Cranial Nerves 1, 5, 7-12 Function: Olfaction Remarkable features: – Peripheral processes act as sensory receptors (the other special sensory nerves have separate Warren L Felton III, MD receptors) Professor and Associate Chair of Clinical – Primary afferent neurons undergo continuous Activities, Department of Neurology replacement throughout life Associate Professor of Ophthalmology – Primary afferent neurons synapse with secondary neurons in the olfactory bulb without synapsing Chair, Division of Neuro-Ophthalmology first in the thalamus (as do all other sensory VCU School of Medicine neurons) – Pathways to cortical areas are entirely ipsilateral 1 2 Crania Nerve I Cranial Nerve I Clinical Testing Pathology Anosmia, hyposmia: loss of or impaired Frequently overlooked in neurologic olfaction examination – 1% of population, 50% of population >60 years Aromatic stimulus placed under each – Note: patients with bilateral anosmia often report nostril with the other nostril occluded, eg impaired taste (ageusia, hypogeusia), though coffee, cloves, or soap taste is normal when tested Note that noxious stimuli such as Dysosmia: disordered olfaction ammonia are not used due to concomitant – Parosmia: distorted olfaction stimulation of CN V – Olfactory hallucination: presence of perceived odor in the absence of odor Quantitative clinical tests are available: • Aura preceding complex partial seizures of eg, University of Pennsylvania Smell temporal lobe origin -
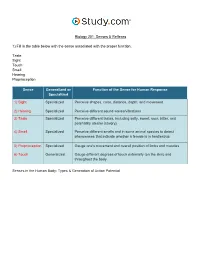
Senses & Reflexes in the Nervous System Visual Worksheet
Biology 201: Senses & Reflexes 1) Fill in the table below with the sense associated with the proper function. Taste Sight Touch Smell Hearing Proprioception Sense Generalized or Function of the Sense for Human Response Specialized 1) Sight Specialized Perceive shapes, color, distance, depth, and movement 2) Hearing Specialized Perceive different sound waves/vibrations 3) Taste Specialized Perceive different tastes, including salty, sweet, sour, bitter, and potentially umami (savory) 4) Smell Specialized Perceive different smells and in some animal species to detect pheromones that indicate whether a female is in heat/estrus 5) Proprioception Specialized Gauge one's movement and overall position of limbs and muscles 6) Touch Generalized Gauge different degrees of touch externally (on the skin) and throughout the body Senses in the Human Body: Types & Generation of Action Potential 2) Label the structures of the eye. Pupil Sclera Retina Lens Iris Cornea The Eye: Structure, Image Detection & Disorders 3) Label the chambers and structures of the eye below. Posterior chamber Muscle Retina Conjunctiva Cornea Sclera Optic nerve Anterior chamber Pupil Choroid layer Blood vessels Lens Iris Vitreous chamber The Eye: Structure, Image Detection & Disorders 4) Label the features of the visual pathway below. Optic chiasm Left cerebral hemisphere Pretectal nucleus Lateral geniculate nucleus of the thalamus Superior colliculus Visual cortex Right cerebral hemisphere The Eye: Structure, Image Detection & Disorders 5) Study the image of the section of the retina below. Label the neural layers. Bipolar cell Cone cell Neural layer Pigmented layer Rod cell Ganglion cell The Eye: Structure, Image Detection & Disorders 6) Label the structures of the nose below. -

Cranial Nerve VIII
Cranial Nerve VIII Color Code Important (The Vestibulo-Cochlear Nerve) Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: ✓ List the nuclei related to vestibular and cochlear nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the vestibular pathways and its main connections. ✓ Describe the auditory pathway and its main connections. Due to the difference of arrangement of the lecture between the girls and boys slides we will stick to the girls slides then summarize the pathway according to the boys slides. Ponto-medullary Sulcus (cerebello- pontine angle) Recall: both cranial nerves 8 and 7 emerge from the ventral surface of the brainstem at the ponto- medullary sulcus (cerebello-pontine angle) Brain – Ventral Surface Vestibulo-Cochlear (VIII) 8th Cranial Nerve o Type: Special sensory (SSA) o Conveys impulses from inner ear to nervous system. o Components: • Vestibular part: conveys impulses associated with body posture ,balance and coordination of head & eye movements. • Cochlear part: conveys impulses associated with hearing. o Vestibular & cochlear parts leave the ventral surface* of brain stem through the pontomedullary sulcus ‘at cerebellopontine angle*’ (lateral to facial nerve), run laterally in posterior cranial fossa and enter the internal acoustic meatus along with 7th (facial) nerve. *see the previous slide Auditory Pathway Only on the girls’ slides 04:14 Characteristics: o It is a multisynaptic pathway o There are several locations between medulla and the thalamus where axons may synapse and not all the fibers behave in the same manner. -

Ear Infections in Children
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Ear Infections in Children What is an ear infection? How can I tell if my child has an ear infection? An ear infection is an inflammation of the middle ear, usually caused by bacteria, that occurs when fluid builds Most ear infections happen to children before they’ve up behind the eardrum. Anyone can get an ear infection, learned how to talk. If your child isn’t old enough to say but children get them more often than adults. Five out of “My ear hurts,” here are a few things to look for: six children will have at least one ear infection by their third } Tugging or pulling at the ear(s) birthday. In fact, ear infections are the most common reason parents bring their child to a doctor. The scientific name for } Fussiness and crying an ear infection is otitis media (OM). } Trouble sleeping What are the symptoms of an } Fever (especially in infants and younger children) ear infection? } Fluid draining from the ear } Clumsiness or problems with balance There are three main types of ear infections. Each has a different combination of symptoms. } Trouble hearing or responding to quiet sounds. } Acute otitis media (AOM) is the most common ear What causes an ear infection? infection. Parts of the middle ear are infected and swollen and fluid is trapped behind the eardrum. This An ear infection usually is caused by bacteria and often causes pain in the ear—commonly called an earache. -

Auditory & Vestibular Systems Steven Mcloon Department of Neuroscience University of Minnesota
Auditory & Vestibular Systems Steven McLoon Department of Neuroscience University of Minnesota 1 The auditory & vestibular systems have many similarities. • The sensory apparatus for both are in canals embedded in the bone of the inner ear. • Receptor cells (hair cells) for both are mechanosensory cells with fine stereocilia. • Information for both is carried into the brain via the vestibulochoclear nerve (cranial nerve VIII). 2 Auditory System • The auditory system detects and interprets sound. • Sound is the vibration of air molecules similar to ripples in water that propagate from a thrown rock. • The sound waves have an amplitude (loudness) and frequency (pitch). 3 Auditory System • Humans can typically hear 20 – 20,000 hertz (cycles per second). 4 Auditory System • Humans can typically hear 20 – 20,000 hertz (cycles per second). http://en.wikipedia.org/wiki/Audio_frequency 5 Auditory System • As a person ages, he/she loses the ability to hear high and low frequencies. 6 Auditory System External ear: • includes the pinna, external auditory meatus (ear canal) and tympanic membrane (ear drum). • The pinna and canal collect sound and guide it to the tympanic membrane. • The tympanic membrane vibrates in response to sound. 7 Auditory System Middle ear: • It is an air filled chamber. • The eustachian tube (auditory tube) connects the middle ear chamber with the pharynx (throat). • Three tiny bones in the chamber transfer the vibration of the tympanic membrane to the oval window of the inner ear. • Two tiny muscles can dampen the movement of the tympanic membrane and bones to protect against a loud sound. 8 Auditory System Inner ear: • The cochlea is a snail shaped tube incased in bone. -

Patient Information – Ear Surgery Instructions
The Oregon Clinic, Plaza ENT Division 5050 NE Hoyt #655, Portland, OR 97213 Phone: 5034882400 Fax: 5032310121 Patient Information – Ear Surgery Instructions Pre- and Post-operative Instructions for Ear Surgery (not including ear tubes) Before Surgery: Many ear surgeries involve manipulation of the eardrum (tympanic membrane), and some require the removal of bone to facilitate the treatment of your ear disease. As with any operation, infection, scarring, and blood clot formation (hematoma) are possible. The facial nerve is at risk for injury or temporary weakness during any ear surgery. Dizziness following surgery may be expected. Hearing loss or ringing in the ear (tinnitus) may be more pronounced. Taste disturbance is not uncommon in certain ear surgeries for a few weeks following surgery and, in a few instances, could be prolonged or permanent. An incision may be made behind your ear, on your earlobe, or behind the pointed cartilage in front of your ear (the tragus). These areas normally heal without problems or obvious scars. Hair around the ear may or may not be shaved. Flying is usually permitted one month after surgery. Swimming may be allowed six weeks after surgery, but check with your doctor first before resuming swimming or other water sports. If your work is not strenuous and depending upon the type of surgery you’ve had, you may return to work 3 to 4 days from the date of surgery. Generally, you will be seen about 2-3 weeks after surgery. This gives your eardrum time to heal before we see you back. Pre-operative Instructions: 1. -

VESTIBULAR SYSTEM (Balance/Equilibrium) the Vestibular Stimulus Is Provided by Earth's Gravity, and Head/Body Movement. Locate
VESTIBULAR SYSTEM (Balance/Equilibrium) The vestibular stimulus is provided by Earth’s gravity, and head/body movement. Located in the labyrinths of the inner ear, in two components: 1. Vestibular sacs - gravity & head direction 2. Semicircular canals - angular acceleration (changes in the rotation of the head, not steady rotation) 1. Vestibular sacs (Otolith organs) - made of: a) Utricle (“little pouch”) b) Saccule (“little sac”) Signaling mechanism of Vestibular sacs Receptive organ located on the “floor” of Utricle and on “wall” of Saccule when head is in upright position - crystals move within gelatinous mass upon head movement; - crystals slightly bend cilia of hair cells also located within gelatinous mass; - this increases or decreases rate of action potentials in bipolar vestibular sensory neurons. Otoconia: Calcium carbonate crystals Gelatinous mass Cilia Hair cells Vestibular nerve Vestibular ganglion 2. Semicircular canals: 3 ring structures; each filled with fluid, separated by a membrane. Signaling mechanism of Semicircular canals -head movement induces movement of endolymph, but inertial resistance of endolymph slightly bends cupula (endolymph movement is initially slower than head mvmt); - cupula bending slightly moves the cilia of hair cells; - this bending changes rate of action potentials in bipolar vestibular sensory neurons; - when head movement stops: endolymph movement continues for slightly longer, again bending the cupula but in reverse direction on hair cells which changes rate of APs; - detects “acceleration” -

Basic Anatomy and Physiology of the Ear 11
1 BASIC ANATOMY AND PHYSIOLOGY OF THE EAR J. Irwin Introduction The ear is a small, complex series of interlinked structures that are involved in both maintenance of normal balance and the sense of hearing. In order to hear, the ear collects the sound waves that arrive as pressure changes in air and converts these into neurochemical impulses that travel along the cochlear- vestibular nerve to the brain. There are both active and passive mechanisms involved in this process.The prime function of the vestibular system is to detect and compensate for movement. This includes the ability to maintain optic fix- ation despite movement and to initiate muscle reflexes to maintain balance. For the purposes of describing structure and function the ear is usually split into four distinct parts. These are the outer ear, the middle ear and the audi- tory and vestibular parts of the inner ear (Figure 1.1). The outer ear This is sometimes known as the external ear and consists of the ear that is visible on the side of the head (the pinna), the external auditory meatus (ear hole) and the ear canal (external auditory canal) that leads to the eardrum (or tympanic membrane). The tympanic membrane has three layers and the outer layer is usually included as part of the outer ear. THE PINNA This is, for the most part, a piece of cartilage covered by skin (Figure 1.2). There is also a fatty earlobe in most people. The skin covering the cartilage is Infection and Hearing Impairment. Edited by V.E. Newton and P.J. -

Intraoperative Neuromonitoring Techniques in the Surgical Management of Acoustic Neuromas
Neurosurg Focus 33 (3):E6, 2012 Intraoperative neuromonitoring techniques in the surgical management of acoustic neuromas TAEMIN OH, B.A.,1 DANIEL T. NAGASAWA, M.D.,1 BRENDAN M. FONG, B.S.,1 ANDY TRANG, B.S.,1 QUINtoN GOPEN, M.D.,3 ANDREW T. PARSA, M.D., PH.D.,2 AND ISAAC YANG, M.D.1,4 Departments of 1Neurosurgery and 3Otolaryngology ENT, David Geffen School of Medicine, University of California, Los Angeles; 4UCLA Jonsson Comprehensive Cancer Center, University of California, Los Angeles; and 2Department of Neurosurgery, UCSF School of Medicine, University of California, San Francisco, California Unfavorable outcomes such as facial paralysis and deafness were once unfortunate probable complications following resection of acoustic neuromas. However, the implementation of intraoperative neuromonitoring during acoustic neuroma surgery has demonstrated placing more emphasis on quality of life and preserving neurological function. A modern review demonstrates a great degree of recent success in this regard. In facial nerve monitoring, the use of modern electromyography along with improvements in microneurosurgery has significantly improved preservation. Recent studies have evaluated the use of video monitoring as an adjunctive tool to further improve outcomes for patients undergoing surgery. Vestibulocochlear nerve monitoring has also been extensively studied, with the most popular techniques including brainstem auditory evoked potential monitoring, electrocochleography, and direct compound nerve action potential monitoring. Among them, direct recording remains the most promising and preferred monitoring method for functional acoustic preservation. However, when compared with postoperative facial nerve function, the hearing preservation is only maintained at a lower rate. Here, the authors analyze the major intraoperative neuromonitoring techniques available for acoustic neuroma resection. -

Reconstruction of Acquired Pinna Defects
Reconstruction of Acquired Pinna Defects Dissertation submitted to THE TAMILNADU Dr. M. G. R. MEDICAL UNIVERSITY In partial fulfillment of the regulations for the award of the degree of MCh (PLASTIC SURGERY) BRANCH III THE TAMIL NADU DR. MGR. MEDICAL UNIVERSITY CHENNAI. AUGUST 2008 DECLARATION I solemnly declare that this dissertation “RECONSTRUCTION OF ACQUIRED PINNA DEFECTS” was prepared by me in the Department of Plastic, Reconstructive and Maxillofacial Surgery, Madras Medical College and Government General Hospital, Chennai under the guidance and supervision of Professor & HOD Department of Plastic, Reconstructive and Maxillofacial Surgery, Madras Medical College and Government General Hospital, Chennai between 2005 and 2008. This dissertation is submitted to the TamilNadu Dr. MGR Medical University, Chennai in partial fulfilment of the University requirements for the award of degree of MCh Plastic Surgery. Place: Chennai Date : CERTIFICATE This is to certify that this dissertation entitled “ RECONSTRUCTION OF ACQUIRED PINNA DEFECTS” is a bonafide record of the research work done by Dr. D.VINOTH KUMAR for the award of MCh Plastic Surgery, under the supervision of Professor & HOD, Plastic Surgery Madras Medical College and Government General Hospital, Chennai between 2005 and 2008. I also certify that this dissertation is the result of the independent work done by candidate. Professor and Head of the Department Dean Plastic, Reconstructive and Maxillofacial Surgery Madras Medical College and Madras Medical College Government General Hospital Chennai – 600 003. Chennai – 600 003. ACKNOWLEDGEMENT I am thankful to the Dean, Madras Medical College and Government General Hospital, Chennai for permitting me to carry out this study. I am very grateful to my teacher and guide Professor V Alamelu M.S., Mch, FICS, FMMS Professor & HOD, Plastic Reconstructive and Maxillofacial Surgery, Madras Medical College, Chennai for her expert guidance and help without which this study would not have been possible. -

The Vestibulocochlear Nerve (VIII)
Diagnostic and Interventional Imaging (2013) 94, 1043—1050 . CONTINUING EDUCATION PROGRAM: FOCUS . The vestibulocochlear nerve (VIII) a,∗ b a F. Benoudiba , F. Toulgoat , J.-L. Sarrazin a Department of neuroradiology, Kremlin-Bicêtre university hospital, 78, rue du Général-Leclerc, 94275 Le Kremlin-Bicêtre, France b Diagnostic and interventional neuroradiology, Laennec hospital, Nantes university hospitals, boulevard Jacques-Monod, Saint-Herblain, 44093 Nantes cedex 1, France KEYWORDS Abstract The vestibulocochlear nerve (8th cranial nerve) is a sensory nerve. It is made up of Cranial nerves; two nerves, the cochlear, which transmits sound and the vestibular which controls balance. It is Pathology; an intracranial nerve which runs from the sensory receptors in the internal ear to the brain stem Vestibulocochlear nuclei and finally to the auditory areas: the post-central gyrus and superior temporal auditory nerve (VIII) cortex. The most common lesions responsible for damage to VIII are vestibular Schwannomas. This report reviews the anatomy and various investigations of the nerve. © 2013 Published by Elsevier Masson SAS on behalf of the Éditions françaises de radiologie. The cochlear nerve Review of anatomy [1] The cochlear nerve has a peripheral sensory origin and follows a centripetal path. It ori- ginates in the cochlear membrane sensory canal, forming the spiral organ (the organ of Corti) and lies on the basilar membrane (Fig. 1). The neuronal fibers of the protoneu- ron connect to the ciliated cells on the spiral lamina (Fig. 2). The axons are grouped together along the axis of the cochlea (modiolus), forming the cochlear nerve which then enters the internal auditory meatus (IAM). Within the internal auditory meatus, the nerve joins the vestibular nerve to form the vestibulocochlear nerve which crosses the cere- bellopontine angle (Figs.