Hunter, R. BIOL 289 Report 7-22-11 FINAL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Monoicous Species Pairs in the Mniaceae (Bryophyta); Morphology, Sexual Condition and Distiribution
ISSN 2336-3193 Acta Mus. Siles. Sci. Natur., 68: 67-81, 2019 DOI: 10.2478/cszma-2019-0008 Published: online 1 July 2019, print July 2019 On the hypothesis of dioicous − monoicous species pairs in the Mniaceae (Bryophyta); morphology, sexual condition and distiribution Timo Koponen On the hypothesis of dioicous − monoicous species pairs in the Mniaceae (Bryophyta); morphology, sexual condition and distiribution. – Acta Mus. Siles. Sci. Natur., 68: 67-81, 2019. Abstract: Some early observations seemed to show that, in the Mniaceae, the doubling of the chromo- some set affects a change from dioicous to monoicous condition, larger size of the gametophyte including larger leaf cell size, and to a wider range of the monoicous counterpart. The Mniaceae taxa are divided into four groups based on their sexual condition and morphology. 1. Dioicous – monoicous counterparts which can be distinguished by morphological characters, 2. Dioicous – monoicous taxa which have no morphological, deviating characters, 3. Monoicous species mostly with diploid chromosome number for which no dioicous counterpart is known, and 4. The taxa in Mniaceae with only dioicous plants. Most of the monoicous species of the Mniaceae have wide ranges, but a few of them are endemics in geographically isolated areas. The dioicous species have either a wide holarctic range or a limited range in the forested areas of temperate and meridional North America, Europe and SE Asia, or in subtropical Asia. Some of the monoicous species are evidently autodiploids and a few of them are allopolyploids from cross-sections of two species. Quite recently, several new possible dioicous – monoicous relationships have been discovered. -

Lord Hill Regional Park Plant Checklist Revised: 2012, 2017 Plants Will Often Be at Other Locations Besides Those Listed
Lord Hill Regional Park Plant Checklist Revised: 2012, 2017 Plants will often be at other locations besides those listed Key to locations: BLP = Beaver Lodge Pond PL = Parking Lot BP = Beaver Pond Trail QT = Quarry Trail BPE = Beaver Pond East Shore RB = Red Barn Trail Cut BPW = Beaver Pond West Shore RL = Red Line Trail BSA = Bike Skills Area RT = River Trail CRP = Cross Roads Pond RTCO = River Tr cut-off EW = E-W trail South of Meetcutter RVT = Riverview TR LTP = Lower Temple Pond Trail SHL = South Shore Hidden Lake M = Many sites including… STPL = South Temple Pond Loop MCT = Meet Cutter Tr TPCT = Temple Pond closed Trail MFP = Midway footpath TPES = Temple Pond East Shore ML = Marsh Lake TPVT = Temple Pond View Trail MT = Main Trail TPST = Trails south of Temple Pond MTC = Main Trail Cutoff TS = Tr. Head Spur NET = NE Trails Area VP =ViewPoint 630 ft NTP = North Temple Pond Loop VPT = View Pt Trail Spur PiT = Pipeline Trail WLT = West Loop Trails PiTTO = Pipeline Turn Out WPS = West Pond Shore Genus: Species: Some Locations: Common Name: Pojar pg: Abies amabilis MT Silver fir 33 Abies grandis MFP?WLT? PiT Grand fir 34 Acer circinatum M, BSA,MCT, BPW,STPL,WLT Vine maple 93 Acer macrophyllum M, VPT,MCT, RT,MFP,STPL Big leaf maple 45 Achillea millefolium PiT Yarrow 279 Achlys triphylla TPCT, BLP Vanilla-leaf 312 Adiantum pedatum NTP, QT Maidenhair fern 425 Agrostis sp. PiT Agrostis grass 367 Agrostis tenuis WLT Colonial bent grass 367 Alisma plantago-aquatica BPW, BPE, CRP Water plantain 337 Alnus rubra M, SHL,MCT,MFP,STPL, WLT Red Alder 44 Amalenchier alnifolia VP, VPT Serviceberry, Saskatoon 72 Anaphalis margaritacea QT Pearly everlasting 304 Anthelia sp. -
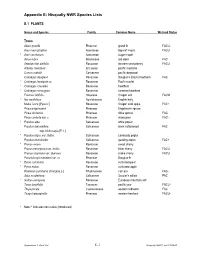
Appendix E Nisqually Species List
Appendix E: Nisqually NWR Species Lists E.1 PLANTS Genus and Species Family Common Name Wetland Status Trees Abies grandis Pinaceae grand fir FACU- Acer macrophyllum Aceraceae big-leaf maple FACU * Acer saccharum Aceraceae sugar maple Alnus rubra Betulaceae red alder FAC Amelanchier alnifolia Rosaceae western serviceberry FACU Arbutus menziesii Ericaceae pacific madrone Cornus nuttallii Cornaceae pacific dogwood Crataegus douglasii Rosaceae Douglas’s (black) hawthorn FAC * Crataegus laevigata cv. Rosaceae Paul's scarlet * Crataegus x lavallei Rosaceae hawthorn * Crataegus monogyna Rosaceae common hawthorn Fraxinus latifolia Oleaceae Oregon ash FACW * Ilex aquifolium Aquifoliaceae English holly Malus fusca [Pyrus f.] Rosaceae Oregon crab apple FAC+ Picea engelmannii Pinaceae Engelmann spruce Picea sitchensis Pinaceae Sitka spruce FAC Pinus contorta var. c. Pinaceae shore pine FAC- * Populus alba Salicaceae white poplar Populus balsamifera Salicaceae black cottonwood FAC ssp. trichocarpa [P. t. ] * Populus nigra var. italica Salicaceae Lombardy poplar Populus tremuloides Salicaceae quaking aspen FAC+ * Prunus avium Rosaceae sweet cherry Prunus emarginata var. mollis Rosaceae bitter cherry FACU Prunus virginiana var. demissa Rosaceae choke cherry FACU Pseudotsuga menziesii var. m. Pinaceae Douglas-fir * Pyrus communis Rosaceae cultivated pear * Pyrus malus Rosaceae cultivated apple Rhamnus purshiana [Frangula p.] Rhamnaceae cascara FAC- Salix scouleriana Salicaceae Scouler’s willow FAC * Sorbus aucuparia Rosaceae European mountain ash Taxus brevifolia Taxaceae pacific yew FACU- Thuja plicata Cupressaceae western redcedar FAC Tsuga heterophylla Pinaceae western hemlock FACU- * Note: * indicates non-native (introduced) Appendix E.1: Plant List E-1 Nisqually NWR Final CCP/EIS Genus and Species Family Common Name Wetland Status Shrubs, Brambles & Vines Acer circinatum Aceraceae vine maple FACU+ Arctostaphylos uva-ursi var. -

Acta Botanica Fennica
T SOCIETAS PRO FAUNA ET FLORA FENNICA ACTA BOTANICA FENNICA 97 Timo Koponen: The East Asiatic species of Plagiomnium sect. Rostrata (Bryophyta) HELSINKI-HELSINGFORS 1972 ACTA BOTANICA FENNICA 1-19 vide Acta Botanica Fennica 20-50. 20-49 vide Acta Botanica Fennica 50-82. SO. HaD.I Luther: Verbreitung und Okologie der hoheren W asserpflanzen im Brack wasser der Ekeniis-Gegend in Siidfinnland. 11. Spezieller Teil. 370 S. (1951). 51. M. R. Droop: On the ecology of Flagellates from some brackish and fresh water rockpools of Finland. 52 pp. (1953). 52. Bans Luther: tlber Vaucheria arrhyncha Heidinger und die Heterokonten-Ordnung Vaucheriales Bohlin. 24 S. (1953). 53. Ernst Biyren: Wasser- und Uferpflanzen aus dem Piiijiinne-Gebiet. 42 S. (1954). 54. Lan Fagerstrom: Viixtgeografiska studier i Stromfors-Pyttis skiirgArd i ostra Nyland med speciellt beaktande av liiviingarna, artantalet samt en del arters for delning och invandring. 296 s. (1954). SS. Bans Luther: tlber Krustenbewuchs an Steinen fliessender Gewiisser, speziell in Siidfinnland. 61 S. (1954). 56. Dmari Bnstich: Notes on the growth of Scotch Pine in Utsjoki in northernmost Finland. 13 pp. (1956). 57. Benrik Skulta Skogsbotaniska studier i SkiirgArdshavet med speciell hiinsyn till forhAllandena i Korpo utsklir. 244 s. (1956). 58. Rolf Griinhlad, Gerald A. Prowse and Arthur M. Scott: Sudanese Desmids. 82 pp. (1958). 59. Max Ton Sehantz: tlber das iitherische 01 beim Kalmus, Acorus calamus L. Phar makognostische Untersuchung. 138 S. (1958). 60. Barald Lindberg: Viixter, kiinda frA.n Norden, i Linnes herbarium. Plantae e septen trione cognitae in herbario Linnaei. 133 pp. (1958). 61. Alvar Palmgren: Studier over havsstrandens vegetation och flora pl Aland. -

Volume 1, Chapter 3-1: Sexuality: Sexual Strategies
Glime, J. M. and Bisang, I. 2017. Sexuality: Sexual Strategies. Chapt. 3-1. In: Glime, J. M. Bryophyte Ecology. Volume 1. 3-1-1 Physiological Ecology. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 3 June 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 3-1 SEXUALITY: SEXUAL STRATEGIES JANICE M. GLIME AND IRENE BISANG TABLE OF CONTENTS Expression of Sex ......................................................................................................................................... 3-1-2 Unisexual and Bisexual Taxa ........................................................................................................................ 3-1-2 Sex Chromosomes ................................................................................................................................. 3-1-6 An unusual Y Chromosome ................................................................................................................... 3-1-7 Gametangial Arrangement ..................................................................................................................... 3-1-8 Origin of Bisexuality in Bryophytes ............................................................................................................ 3-1-11 Monoicy as a Derived/Advanced Character? ........................................................................................ 3-1-11 Multiple Reversals .............................................................................................................................. -

TAS3 Mir390-Dependent Loci in Non-Vascular Land Plants: Towards a Comprehensive Reconstruction of the Gene Evolutionary History
TAS3 miR390-dependent loci in non-vascular land plants: towards a comprehensive reconstruction of the gene evolutionary history Sergey Y. Morozov1, Irina A. Milyutina1, Tatiana N. Erokhina2, Liudmila V. Ozerova3, Alexey V. Troitsky1 and Andrey G. Solovyev1,4 1 Belozersky Institute of Physico-Chemical Biology, Moscow State University, Moscow, Russia 2 Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Science, Moscow, Russia 3 Tsitsin Main Botanical Garden, Russian Academy of Science, Moscow, Russia 4 Institute of Molecular Medicine, Sechenov First Moscow State Medical University, Moscow, Russia ABSTRACT Trans-acting small interfering RNAs (ta-siRNAs) are transcribed from protein non- coding genomic TAS loci and belong to a plant-specific class of endogenous small RNAs. These siRNAs have been found to regulate gene expression in most taxa including seed plants, gymnosperms, ferns and mosses. In this study, bioinformatic and experimental PCR-based approaches were used as tools to analyze TAS3 and TAS6 loci in transcriptomes and genomic DNAs from representatives of evolutionary distant non-vascular plant taxa such as Bryophyta, Marchantiophyta and Anthocero- tophyta. We revealed previously undiscovered TAS3 loci in plant classes Sphagnopsida and Anthocerotopsida, as well as TAS6 loci in Bryophyta classes Tetraphidiopsida, Polytrichopsida, Andreaeopsida and Takakiopsida. These data further unveil the evolutionary pathway of the miR390-dependent TAS3 loci in land plants. We also identified charophyte alga sequences coding for SUPPRESSOR OF GENE SILENCING 3 (SGS3), which is required for generation of ta-siRNAs in plants, and hypothesized that the appearance of TAS3-related sequences could take place at a very early step in Submitted 19 February 2018 evolutionary transition from charophyte algae to an earliest common ancestor of land Accepted 28 March 2018 plants. -

A CHECKLIST of MONTANA MOSSES (1880–2018) January 3, 2020
A CHECKLIST OF MONTANA MOSSES (1880–2018) January 3, 2020 Joe C. Elliott Conservation Biology Research, Missoula, Montana Andrea K. Pipp Montana Natural Heritage Program, 1515 E Sixth Ave, Helena, Montana 59601 INTRODUCTION Montana has one of the richest recorded moss floras of the western United States (Eckel et al. 1997), even though large areas of the state remain under surveyed. The Flora of North America (FNA) volumes 27 (2007) and 28 (2014) include 1,402 species found in the continental United States, Canada, Greenland, and St. Pierre and Miquelon, of which 508 species have been recorded in Montana. Including varieties and subspecies, Montana has 522 moss taxa. The rich moss flora is due to the habitat and climatic diversity across the state and a long history of bryological exploration that began in the late 1800s. This checklist is a revision to the second preliminary checklist (Elliott 1993), which listed 408 taxa. The substantial increase in the number of moss taxa since 1993 indicates that, as in much of the western United States, our knowledge of the Montana moss flora continues to expand with increased field and herbarium studies. The discovery of mosses in eastern North America appears to be reaching saturation, but this is not true for western North America, where the accumulation of new species has continued to rise steeply over the last three decades (Carter et al. 2016). Another publication titled the “History, Biogeography, and Species of Montana Mosses (1880-2018)” will be published in Volume 36, Issue 2 of Evansia, a peer-reviewed quarterly of The American Bryological and Lichenological Society (2019). -
Other Studies—Shephard (1995) Describes a Similar Salix Barclayi/Carex Sitchensis Carex Sitchensis (Barclay Willow/Sitka Sedge) C.T
Figure 23—Salix barclayi/Carex sitchensis c.t. on distal outwash below the Sheridan Glacier. Salix barclayi/ Other studies—Shephard (1995) describes a similar Salix barclayi/Carex sitchensis Carex sitchensis (Barclay willow/Sitka sedge) c.t. from the Yakutat Foreland in the Tongass National Community Type Forest. The Copper River Delta and the foreland are the only places this community Barclay Willow/ Sitka Sedge type has been reported in the state, although various Salix barclayi types are Community Type described from southeast and south-central Alaska (Viereck and others 1992). SALBAR/CARSIT G3; S3 Vegetation—The overstory is dominated by Salix barclayi (Barclay willow), and Myrica gale (sweetgale) is a common associate (fig. 23). Height of the tallest shrub layer ranges from 3 to 8 feet. Salix barclayi was heavily browsed within several plots. The diagnostic understory species Carex sitchensis (Sitka sedge), Menyanthes trifoliata (buckbean), and Potentilla palustris (marsh fivefinger) dominate the herbaceous layer. Bryophyte cover is highly variable. The following tabulation lists the species that occur in more than 50 percent of the sites (50 percent constancy) and gives the percentage of constancy, average percentage of canopy cover for sites in which they occur, and range of cover values (number of sites sampled = 8; species richness = 32): 121 Species Constancy Average Range - - - - - - - - - - Percent - - - - - - - - - - Shrubs: Alnus crispa var. sinuata 50 3 0-10 Myrica gale 63 31 0-50 Salix barclayi 100 40 0-60 Salix commutata 50 21 0-30 Forbs: Equisetum palustre 63 17 0-30 Menyanthes trifoliata 13 20 0-20 Potentilla palustris 50 18 0-30 Rubus arcticus 63 6 0-10 Trientalis europaea 63 1 0-3 Graminoids: Calamagrostis canadensis 63 17 0-60 Carex sitchensis 100 27 3-50 Environmental characteristics—This is a major type found on the uplifted marshes and an incidental type on the outwash plains. -

Chapter 2-2 Life Cycles: Surviving Change
Glime, J. M. 2017. Life Cycles: Surviving Change. Chapt. 2-2. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. 2-2-1 Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 9 April 2021 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 2-2 LIFE CYCLES: SURVIVING CHANGE TABLE OF CONTENTS The General Bryobiotina Life Cycle................................................................................................................... 2-2-2 Dominant Generation.......................................................................................................................................... 2-2-3 The Life Cycle .................................................................................................................................................... 2-2-3 Life Cycle Controls........................................................................................................................................... 2-2-13 Generation Time................................................................................................................................................ 2-2-13 Importance................................................................................................................................................. 2-2-16 Longevity and Totipotency ............................................................................................................................... 2-2-16 Summary .......................................................................................................................................................... -

Bryophyte Biogeography
Critical Reviews in Plant Sciences ISSN: 0735-2689 (Print) 1549-7836 (Online) Journal homepage: http://www.tandfonline.com/loi/bpts20 Bryophyte Biogeography J. Patiño & A. Vanderpoorten To cite this article: J. Patiño & A. Vanderpoorten (2018): Bryophyte Biogeography, Critical Reviews in Plant Sciences, DOI: 10.1080/07352689.2018.1482444 To link to this article: https://doi.org/10.1080/07352689.2018.1482444 Published online: 06 Sep 2018. Submit your article to this journal Article views: 58 View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=bpts20 CRITICAL REVIEWS IN PLANT SCIENCES https://doi.org/10.1080/07352689.2018.1482444 Bryophyte Biogeography J. Patino~ a,b,c and A. Vanderpoortend aInstituto de Productos Naturales & Agrobiologıa (IPNA-CSIC), Island Ecology and Evolution Research Group, La Laguna, Tenerife, Spain; bDepartment of Environmental Science, Policy and Management, University of California, Berkeley, CA, USA; cDepartamento de Botanica, Ecologıa y Fisiologıa Vegetal, Universidad de La Laguna, La Laguna, Spain; dInstitute of Botany, University of Liege, Liege, Belgium ABSTRACT KEYWORDS Bryophytes include about 20,000 species characterized by their poikilohydric condition, high Distribution range; long-distance dispersal capacities, and cold tolerance. Despite these specific life-history traits, endemism; genetic large-scale biogeographic patterns in bryophytes are consistent with those observed in other structure; latitudinal groups, -

Copper River Delta
United States Department of Agriculture Classification of Community Forest Service Pacific Northwest Types, Successional Research Station General Technical Sequences, and Report PNW-GTR-469 March 2000 Landscapes of the Copper River Delta, Alaska Keith Boggs Author Keith Boggs is the staff vegetation ecologist, Alaska Natural Heritage Program, Environment and Natural Resources Institute, School of Arts and Sciences, University of Alaska Anchorage, 707 A Street, Anchorage, AK 99501. This publication is the result of a cooperative study between the U.S. Department of Agriculture, Forest Service, Chugach National Forest and the Alaska Natural Heritage Program. Abstract Boggs, Keith. 2000 Classification of community types, successional sequences, and landscapes of the Copper River Delta, Alaska. Gen. Tech. Rep. PNW-GTR-469. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 244 p. A classification of community types, successional sequences, and landscapes is pre- sented for the piedmont of the Copper River Delta. The classification was based on a sampling of 471 sites. A total of 75 community types, 42 successional sequences, and 6 landscapes are described. The classification of community types reflects the existing vegetation communities on the landscape. The distribution, vegetation composition and structure, soils, and successional status of each community are discussed. The community types were placed within successional sequences reflecting their succes- sional trends. Geomorphic and soil development were closely aligned with vegetation succession on the study area and, consequently, are described in detail. Each succes- sional sequence was named after the oldest community type identified in the sequence and the landscape on which it occurs. -

Downloaded the Available Rcbl and Trnl-F Sequences from the Genbank Database ( for Each Species
DETECTING THE PHYLOGENETIC SIGNAL OF GLACIAL REFUGIA IN A BRYODIVERSITY HOTSPOT OUTSIDE THE TROPICS by Ernest Ting Yu Wu BSAB, University of British Columbia, 2018 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in The Faculty of Graduate and Postdoctoral Studies (Forestry) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) September 2020 © Ernest Ting Yu Wu, 2020 The following individuals certify that they have read, and recommend to the Faculty of Graduate and Postdoctoral Studies for acceptance, a thesis entitled: Detecting the Phylogenetic Signal of Glacial Refugia in a Byrodiversity Hotspot Outside the Tropics submitted by Ernest T. Y. Wu in partial fulfillment of the requirements for the degree of Master of Science in Forestry Examining Committee: Dr. T. Jonathan Davies, Professor, Botany, UBC Supervisor Dr. Robert D. Guy, Professor, Forest & Conservation Sciences, UBC Supervisory Committee Member Ms. Linda Jennings, Collections curator, Beaty Biodiversity Museum , UBC Supervisory Committee Member Dr. Quentin C. B. Cronk, Professor, Botany, UBC Additional Examiner ii Abstract Glacial refugia have likely been important in shaping diversity gradients outside the tropics. However, the biogeographical histories of most species within glacial refugia remain unclear. In this thesis, I examine the geographic range structure and phylogenetic attributes of the mosses of Haida Gwaii, a putative glacial refugium and ‘hotspot’ of moss diversity off the northwest coast of British Columbia. I show that many species have widespread, but disjunct distributions, typically with few close relatives on the islands. I suggest that these features reflect the imprint of glacial history, whereby species within refugia represent isolated populations of previously more widespread species that may have diversified elsewhere.