Lab #3 Class Bryopsida
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
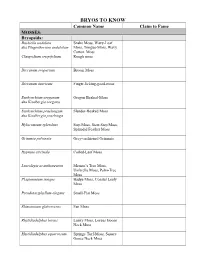
Bryo's to Know Table
BRYOS TO KNOW Common Name Claim to Fame MOSSES: Bryopsida: Buckiella undulata Snake Moss, Wavy-Leaf aka Plagiothecium undulatum Moss, Tongue-Moss, Wavy Cotton, Moss Claopodium crispifolium Rough moss Dicranum scoparium Broom Moss Dicranum tauricum Finger-licking-good-moss Eurhynchium oreganum Oregon Beaked-Moss aka Kindbergia oregana Eurhynchium praelongum Slender-Beaked Moss aka Kindbergia praelonga Hylocomium splendens Step Moss, Stair-Step Moss, Splendid Feather Moss Grimmia pulvinata Grey-cushioned Grimmia Hypnum circinale Coiled-Leaf Moss Leucolepis acanthoneuron Menzie’s Tree Moss, Umbrella Moss, Palm-Tree Moss Plagiomnium insigne Badge Moss, Coastal Leafy Moss Pseudotaxiphyllum elegans Small-Flat Moss Rhizomnium glabrescens Fan Moss Rhytidiadelphus loreus Lanky Moss, Loreus Goose Neck Moss Rhytidiadelphus squarrosum Springy Turf-Moss, Square Goose Neck Moss Rhytidiadelphus triquetrus Electrified Cat-Tail Moss, Goose Necked Moss Rhytidiopsus robusta Robust mountain moss Schistostega pennata Goblin’s Gold, Luminous Moss Polytrichopsida: Atrichum Atrichum Moss , Crane’s Bill Moss (for Atrichum selwynii) Pogonatum contortum Contorted Pogonatum Moss Polytrichum commune Common Hair Cap Moss Polytrichum piliferum Bristly Haircap Moss Andreaeopsida Andreaea nivalis Granite moss, Lantern moss, Snow Rock Moss Sphagnopsida: Sphagnum capillifolium Red Bog Moss, Small Red Peat Moss Sphagnum papillosum Fat Bog Moss, Papillose sphagnum Sphagnum squarrosum Shaggy Sphagnum, Spread- Leaved Peat Moss Takakiopsida: Takakia lepidoziooides Impossible -

Revision and Checklist of the Moss Families Bartramiaceae and Mniaceae in Vietnam Timo KOPONEN1, Thanh-Luc NGUYEN2, Thien-Tam L
Hattoria 10: 69–107. 2019 Revision and checklist of the moss families Bartramiaceae and Mniaceae in Vietnam Timo KOPONEN1, Thanh-Luc NGUYEN2, Thien-Tam LUONG3, 4 & Sanna HUTTUNEN4 1 Finnish-Chinese Botanical Foundation, Mailantie 109, FI-08800 Lohja, Finland & Finnish Museum of Natural History, Botany Unit (bryology), P.O. Box 7 (Unioninkatu 4), FI-00014 University of Helsinki, Finland 2 Southern Institute of Ecology, Vietnam Academy of Science and Technology, 1 Mac Dinh Chi, District 1, Ho Chi Minh City, Vietnam 3 University of Science, Vietnam National University Ho Chi Minh City, 227 Nguyen Van Cu, District 5, Ho Chi Minh City, Vietnam 4 Herbarium (TUR), Biodiversity Unit, FI 20014 University of Turku, Finland Author for correspondence: Thanh-Luc NGUYEN, [email protected] Abstract The genera Fleischerobryum Loeske and Philonotis Brid. of the Bartramiaceae and the family Mniaceae (excluding Pohlia Hedw.) are revised for Vietnam, based on specimens studied and literature reports. Four species are added to the flora: Orthomnion javense (M.Fleisch.) T.J.Kop., Philonotis asperifolia Mitt., P. laii T.J.Kop., P. speciosa (Griff.) Mitt. syn. nov. (based on P. mercieri Paris & Broth.), and Plagiomnium wui (T.J.Kop.) Y.J.Yi & S.He. Eight species are excluded from the flora. Two taxa are considered doubtful. The flora now includes one species of Fleischerobryum, eight species of Philonotis, one species of Mnium Hedw. (doubtful), three species of Orthomnion Wills. and five species of Plagiomnium (one doubtful). The 15 species are divided into phytogeographical elements. Eight belong to the Southeast Asiatic temperate to meridional element, and seven to the Southeast Asiatic meridional to subtropical element. -

Coptis Trifolia Conservation Assessment
CONSERVATION ASSESSMENT for Coptis trifolia (L.) Salisb. Originally issued as Management Recommendations December 1998 Marty Stein Reconfigured-January 2005 Tracy L. Fuentes USDA Forest Service Region 6 and USDI Bureau of Land Management, Oregon and Washington CONSERVATION ASSESSMENT FOR COPTIS TRIFOLIA Table of Contents Page List of Tables ................................................................................................................................. 2 List of Figures ................................................................................................................................ 2 Summary........................................................................................................................................ 4 I. NATURAL HISTORY............................................................................................................. 6 A. Taxonomy and Nomenclature.......................................................................................... 6 B. Species Description ........................................................................................................... 6 1. Morphology ................................................................................................................... 6 2. Reproductive Biology.................................................................................................... 7 3. Ecological Roles ............................................................................................................. 7 C. Range and Sites -

Fossil Mosses: What Do They Tell Us About Moss Evolution?
Bry. Div. Evo. 043 (1): 072–097 ISSN 2381-9677 (print edition) DIVERSITY & https://www.mapress.com/j/bde BRYOPHYTEEVOLUTION Copyright © 2021 Magnolia Press Article ISSN 2381-9685 (online edition) https://doi.org/10.11646/bde.43.1.7 Fossil mosses: What do they tell us about moss evolution? MicHAEL S. IGNATOV1,2 & ELENA V. MASLOVA3 1 Tsitsin Main Botanical Garden of the Russian Academy of Sciences, Moscow, Russia 2 Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia 3 Belgorod State University, Pobedy Square, 85, Belgorod, 308015 Russia �[email protected], https://orcid.org/0000-0003-1520-042X * author for correspondence: �[email protected], https://orcid.org/0000-0001-6096-6315 Abstract The moss fossil records from the Paleozoic age to the Eocene epoch are reviewed and their putative relationships to extant moss groups discussed. The incomplete preservation and lack of key characters that could define the position of an ancient moss in modern classification remain the problem. Carboniferous records are still impossible to refer to any of the modern moss taxa. Numerous Permian protosphagnalean mosses possess traits that are absent in any extant group and they are therefore treated here as an extinct lineage, whose descendants, if any remain, cannot be recognized among contemporary taxa. Non-protosphagnalean Permian mosses were also fairly diverse, representing morphotypes comparable with Dicranidae and acrocarpous Bryidae, although unequivocal representatives of these subclasses are known only since Cretaceous and Jurassic. Even though Sphagnales is one of two oldest lineages separated from the main trunk of moss phylogenetic tree, it appears in fossil state regularly only since Late Cretaceous, ca. -

Riparian Bryophytes of the H. J. Andrews Experimental Forest in the Western Cascades, Oregon
The Bryologist 99(2), pp. 226-235 Copyright © 1996 by the American Bryological and Lichenological Society, Inc. Riparian Bryophytes of the H. J. Andrews Experimental Forest in the Western Cascades, Oregon BENGT GUNNAR JONSSON I Department of Forest Science, Oregon State University, Corvallis, OR 97331 Abstract. The knowledge of the distribution and habitat demands for bryophytes in the Pacific Northwest is scarce, and few published quantitative accounts of the flora are present. The present paper includes habitat description, elevational range, substrate preference, and frequency esti- mates for more than 130 riparian mosses and liverworts found in old-growth Pseudotsuga-Tsuga forests of the H. J. Andrews Experimental Forest, Oregon. The data are based on 360 samples distributed among 42 sites covering 1st to 5th order streams and 420 to 1250 m. TWINSPAN analysis resulted in 6 sample groups, representing samples from different elevations, geomorphic surfaces, and stream sizes. The most common mosses are Eurhynchium oreganum, Isothecium stoloniferum, Hypnum circinale, and Dicranum fuscescens. Among the hepatics Scapania bolan- deri, Cephalozia lunulifolia, and Porella navicularis are the most abundant species. Most species are rare at both site and sampte gtot (ever; and this is especially true for acrocarps where more than one-third of the observed species occurred in only one or two sites orland samples. Four of the occurring species (i.e., Antitrichia curtipendula, Buxbaumia piperi, Douinia ovata, and Ptili- dium californicum) are listed for special management and/or regional surveys. Bryophytes constitute an important and conspic- a wide range of different substrates, disturbance uous component of old-growth forests in the Pacific patterns, and a moist microclimate are important Northwest (Lesica et al. -

Monoicous Species Pairs in the Mniaceae (Bryophyta); Morphology, Sexual Condition and Distiribution
ISSN 2336-3193 Acta Mus. Siles. Sci. Natur., 68: 67-81, 2019 DOI: 10.2478/cszma-2019-0008 Published: online 1 July 2019, print July 2019 On the hypothesis of dioicous − monoicous species pairs in the Mniaceae (Bryophyta); morphology, sexual condition and distiribution Timo Koponen On the hypothesis of dioicous − monoicous species pairs in the Mniaceae (Bryophyta); morphology, sexual condition and distiribution. – Acta Mus. Siles. Sci. Natur., 68: 67-81, 2019. Abstract: Some early observations seemed to show that, in the Mniaceae, the doubling of the chromo- some set affects a change from dioicous to monoicous condition, larger size of the gametophyte including larger leaf cell size, and to a wider range of the monoicous counterpart. The Mniaceae taxa are divided into four groups based on their sexual condition and morphology. 1. Dioicous – monoicous counterparts which can be distinguished by morphological characters, 2. Dioicous – monoicous taxa which have no morphological, deviating characters, 3. Monoicous species mostly with diploid chromosome number for which no dioicous counterpart is known, and 4. The taxa in Mniaceae with only dioicous plants. Most of the monoicous species of the Mniaceae have wide ranges, but a few of them are endemics in geographically isolated areas. The dioicous species have either a wide holarctic range or a limited range in the forested areas of temperate and meridional North America, Europe and SE Asia, or in subtropical Asia. Some of the monoicous species are evidently autodiploids and a few of them are allopolyploids from cross-sections of two species. Quite recently, several new possible dioicous – monoicous relationships have been discovered. -

Plant Life MagillS Encyclopedia of Science
MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE Volume 4 Sustainable Forestry–Zygomycetes Indexes Editor Bryan D. Ness, Ph.D. Pacific Union College, Department of Biology Project Editor Christina J. Moose Salem Press, Inc. Pasadena, California Hackensack, New Jersey Editor in Chief: Dawn P. Dawson Managing Editor: Christina J. Moose Photograph Editor: Philip Bader Manuscript Editor: Elizabeth Ferry Slocum Production Editor: Joyce I. Buchea Assistant Editor: Andrea E. Miller Page Design and Graphics: James Hutson Research Supervisor: Jeffry Jensen Layout: William Zimmerman Acquisitions Editor: Mark Rehn Illustrator: Kimberly L. Dawson Kurnizki Copyright © 2003, by Salem Press, Inc. All rights in this book are reserved. No part of this work may be used or reproduced in any manner what- soever or transmitted in any form or by any means, electronic or mechanical, including photocopy,recording, or any information storage and retrieval system, without written permission from the copyright owner except in the case of brief quotations embodied in critical articles and reviews. For information address the publisher, Salem Press, Inc., P.O. Box 50062, Pasadena, California 91115. Some of the updated and revised essays in this work originally appeared in Magill’s Survey of Science: Life Science (1991), Magill’s Survey of Science: Life Science, Supplement (1998), Natural Resources (1998), Encyclopedia of Genetics (1999), Encyclopedia of Environmental Issues (2000), World Geography (2001), and Earth Science (2001). ∞ The paper used in these volumes conforms to the American National Standard for Permanence of Paper for Printed Library Materials, Z39.48-1992 (R1997). Library of Congress Cataloging-in-Publication Data Magill’s encyclopedia of science : plant life / edited by Bryan D. -

About the Book the Format Acknowledgments
About the Book For more than ten years I have been working on a book on bryophyte ecology and was joined by Heinjo During, who has been very helpful in critiquing multiple versions of the chapters. But as the book progressed, the field of bryophyte ecology progressed faster. No chapter ever seemed to stay finished, hence the decision to publish online. Furthermore, rather than being a textbook, it is evolving into an encyclopedia that would be at least three volumes. Having reached the age when I could retire whenever I wanted to, I no longer needed be so concerned with the publish or perish paradigm. In keeping with the sharing nature of bryologists, and the need to educate the non-bryologists about the nature and role of bryophytes in the ecosystem, it seemed my personal goals could best be accomplished by publishing online. This has several advantages for me. I can choose the format I want, I can include lots of color images, and I can post chapters or parts of chapters as I complete them and update later if I find it important. Throughout the book I have posed questions. I have even attempt to offer hypotheses for many of these. It is my hope that these questions and hypotheses will inspire students of all ages to attempt to answer these. Some are simple and could even be done by elementary school children. Others are suitable for undergraduate projects. And some will take lifelong work or a large team of researchers around the world. Have fun with them! The Format The decision to publish Bryophyte Ecology as an ebook occurred after I had a publisher, and I am sure I have not thought of all the complexities of publishing as I complete things, rather than in the order of the planned organization. -

Volume 1, Chapter 2-7: Bryophyta
Glime, J. M. 2017. Bryophyta – Bryopsida. Chapt. 2-7. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook 2-7-1 sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 10 January 2019 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 2-7 BRYOPHYTA – BRYOPSIDA TABLE OF CONTENTS Bryopsida Definition........................................................................................................................................... 2-7-2 Chromosome Numbers........................................................................................................................................ 2-7-3 Spore Production and Protonemata ..................................................................................................................... 2-7-3 Gametophyte Buds.............................................................................................................................................. 2-7-4 Gametophores ..................................................................................................................................................... 2-7-4 Location of Sex Organs....................................................................................................................................... 2-7-6 Sperm Dispersal .................................................................................................................................................. 2-7-7 Release of Sperm from the Antheridium..................................................................................................... -

Phytochemical Studies of the Moss Species Plagiomnium Elatum and Plagiomnium Cuspidatum
journ. Hattori Bot. Lab. No. 67: 377- 382 (Dec. 1989) PHYTOCHEMICAL STUDIES OF THE MOSS SPECIES PLAGIOMNIUM ELATUM AND PLAGIOMNIUM CUSPIDATUM 1 S. ANHUT , T. SEEGER 1, J. B IEHL 1 , H. D . ZINSMEISTER 1 AND H . GEIGER 2 ABSTRACT. The flavonoid pattern of P/agiomnium cuspidatum and P. e/atum was evaluated. Fifteen dif ferent flavones, mainly flavone glycosides were isolated. The new natural compounds Elatin; 5-0H-Amento flavone; 2,3-Dihydro-5' -hydroxyamentoflavone, 2,3-Dihydro-5' -hydroxyrobustaflavone and 2,3-Dihydro- 5',3"'-dihydroxyamentoflavone were amongst them. The phytochemical relevance of these results is briefly discussed. INTRODUCTION The fi rst phytochemical studies on Mnium species was done by Kozlowski (1921). He detected Saponarin in Mnium cu:,pidatum, based on a chemical reaction with iodinepotassium iodide solution. Melchert & Alston (1965) reported on the occurrence of flavon C-glycosides in Mnium affine, McClure and Miller (1967) offlavonoids in Mnium cu.lpidatum. Koponen and Nilsson (1977) compared the flavonoid patterns of Plagiomnium elfipticum, P. medium, P. insigne, P. affine, P. tezukae, P. ciliare, P. ela/um, P. drummondii, P. japonicum, P. acutum and P. cuspidatum. Vandekerkhove (1977) isolated two f1avonoids from Mnium undulatum and characterized them as saponarin and an apigenin-6,8 di-C-glycoside. Further C- and C-O-glycosides from the same species were isolated and identified by Osterdahl (1979). Freitag et at. (1986) isolated two new isoorientin-O-diglycosides from P. affine. According to the "Generic revision of Mniaceae" (Koponen, 1968) all mentioned species were allocated to the genus Plagiomnium. The revised classification of the family Mniaceae resulted in four tribes with ten genera on the basis of morphological and karyological data. -

The Biflavonoid Pattern of Rhytidiadelphus Squarrosus (Hedw.) Warnst
The Biflavonoid Pattern of Rhytidiadelphus squarrosus (Hedw.) Warnst. Tassilo Seeger, Hans Dietmar Zinsmeister FB 13, Botanik, Universität des Saarlandes, D-6600 Saarbrücken, Bundesrepublik Deutschland Hans Geiger Institut für Chemie der Universität Hohenheim, D-7000 Stuttgart 70, Bundesrepublik Deutschland Z. Naturforsch. 45c, 583-586 (1990); received January 22, 1990 Dedicated to Dr. Ella Campbell, Massey University, Palmerston North, New Zealand, on the occasion of her 80th birthday Mosses, Hylocomiaceae, Rhytidiadelphus squarrosus, Biflavonoids, 5'-Hydroxyrobustaflavone From Rhytidiadelphus squarrosus the hitherto unknown biflavone 5'-hydroxyrobusta- flavone and the three known biflavonoids 5'-hydroxyamentoflavone,5',3"'-dihydroxyamento- flavone and 2,3-dihydro-5'-hydroxyamentoflavone have been isolated. Introduction 0H Various biflavonoids have been isolated so far from eleven species of seven different moss fami lies, namely Dicranaceae [1-3], Grimmiaceae [4], Bryaceae [5], Mniaceae [4,6], Bartramiaceae [7], Leucodontaceae [4] and Hylocomiaceae [4,8 ]. The most common moss biflavonoids are di mers of luteolin and their 2,3- or 2",3"-dihydro-de- rivatives. Biflavonoids containing monomers oth 5'-Hydroxyamentoflavone (1) R = H 5'-3"'-Dihydroxyamentoflavone (2) R = OH er than luteolin or eriodictyol (= 2,3-dihydroluteo- lin) have been found until now only in species of Bryaceae and Mniaceae [9], both belonging to the 0H suborder Bryineae Fleisch. [10]. Here the isolation of three biflavonoids, containing an apigenin moiety, from Rhytidiadelphus squarrosus which belongs to the Hypnineae Fleisch. [10] is reported. Results and Discussion From Rhytidiadelphus squarrosus four biflavon 5'-Hydroxyrobustaflavone (3) R = H oids 1, 2, 3 and 4 were isolated. 1, 2 and 4 are 5',3"'-Dihydroxyrobustaflavone (5) R = OH known compounds. -

Rhytidiadelphus Squarrosus
Rhytidiadelphus squarrosus Britain 1990–2013 2416 1950–1989 286 pre-1950 5 Ireland 1990–2013 590 1950–1989 180 pre-1950 4 robust moss with a wide ecological tolerance, occurring by heavy grazing and mowing, but is invariably absent from Aon all but the most acid soils in a variety of grassy reseeded and agriculturally improved fields. Altitudinal habitats, including sheep pastures, roadside and trackside range: 0–1170 m. verges, light woodland and scrub, dunes, streamsides, ditches and marshes. It also occurs among ericaceous shrubs Dioicous; capsules are rare in the lowlands but more and in flushed turf on heath and moorland. It is a very frequent in the north and west, mature in winter and early common species of lawns (where it attracts the attention and spring. Its ubiquity is remarkable, considering the rarity of often the disapproval of gardeners!), as well as churchyards, capsules and lack of gemmae. Presumably it is dispersed parkland and other places that are regularly mown and vegetatively by stem and leaf fragments. not heavily fertilised, and likewise it occurs in low-lying unimproved pastures. Regular associates include Lophocolea Plants from woodland often have a lax habit resembling bidentata, Brachythecium rutabulum, Calliergonella cuspidata, R. subpinnatus and the separation of the two species is Kindbergia praelonga and Pseudoscleropodium purum. It sometimes difficult. It is possible that there may be a few ascends to high altitudes in mountain grassland and Nardus recording errors in woodland habitats, but with little impact snowbeds, where associates include Hylocomium splendens, on the overall accuracy of the map. Pleurozium schreberi and Rhytidiadelphus loreus.