Study of Anxiolytic Activity in Mice Using Vacha Churnam (Rasayana Preparation- Ayurvedic System of Medicine)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abuse-Proof Solid Pharmaceutical Composition
(19) TZZ¥¥ ¥_T (11) EP 3 329 939 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 06.06.2018 Bulletin 2018/23 A61K 47/02 (2006.01) A61K 47/36 (2006.01) A61K 9/16 (2006.01) A61K 31/485 (2006.01) (2006.01) (21) Application number: 17204525.4 A61K 31/192 (22) Date of filing: 29.11.2017 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • BOSCHETTI, Silvia GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO I-38060 Aldeno (TN) (IT) PL PT RO RS SE SI SK SM TR • ROSSI, Massimiliano Designated Extension States: I-38121 Trento (IT) BA ME • BENFENATI, Diego Designated Validation States: I-38052 Caldonazzo (TN) (IT) MA MD • POJER, Alessandro I-38092 Altavalle (TN) (IT) (30) Priority: 02.12.2016 IT 201600122469 (74) Representative: Allaix, Roberto (71) Applicant: E-Pharma Trento S.p.A. PGA S.p.A. 38123 Trento (IT) Via Mascheroni, 31 20145 Milano (IT) (54) ABUSE-PROOF SOLID PHARMACEUTICAL COMPOSITION (57) The present invention relates to an abuse-proof solid pharmaceutical composition comprising an active ingre- dient with potential for abuse, silica in an amount of from 10 mg to 1000 mg, guar flour in an amount of from 100 mg to 300 mg, and a water soluble diluent in an amount of from 500 mg to 5000 mg. EP 3 329 939 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 329 939 A1 Description FIELD OF THE INVENTION 5 [0001] The present invention relates to an abuse-proof solid pharmaceutical composition, in particular a solid phar- maceutical composition, such as, for example a granulate or a tablet, capable of solubilizing in a glass of water (about 100 ml), but of forming a not injectable gel and/or viscous solution if added to the amount of water contained in a hypodermic syringe (about 10 ml). -

The Effect of the Roots of Empathy Program on the Use of Psychotropic Medications Among Youth in Manitoba
The effect of the Roots of Empathy program on the use of psychotropic medications among youth in Manitoba by Lindsey Dahl A Thesis submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fulfilment of the requirements of the degree of MASTER OF SCIENCE Department of Community Health Sciences, Rady Faculty of Health Sciences, Max Rady College of Medicine University of Manitoba Winnipeg Copyright © 2017 by Lindsey Dahl Abstract Background: Psychotropic medications prescriptions to youth have increased. Roots of Empathy (ROE) is a social and emotional learning program that may influence the use of psychotropic medication. Methods: Administrative data was analyzed in a matched sample of children who received ROE during 2002/03 to 2012/13. Kaplan-Meier survival curves and Cox proportional hazard models were used to estimate the association between ROE and psychotropic medication dispensations. Results: Few significant differences were observed. Children who received ROE in kindergarten to grade 3 had a lower adjusted hazard for an anxiolytic dispensation. Children who received ROE in grade 7 to 8 had a higher hazard for an antipsychotic dispensation. Males who received the program had an increased hazard for an antipsychotic dispensation. Conclusion: There was no consistent differences in the likelihood of being dispensed a psychotropic medication between children who received ROE and children who did not in Manitoba. ii Acknowledgments I would like to first thank my thesis advisor Dr. Randy Fransoo of the Department of Community Health Sciences, Rady Faculty of Health Sciences, Max Rady College of Medicine at the University of Manitoba. Right from our initial meeting, Dr. -

Pharmacology on Your Palms CLASSIFICATION of the DRUGS
Pharmacology on your palms CLASSIFICATION OF THE DRUGS DRUGS FROM DRUGS AFFECTING THE ORGANS CHEMOTHERAPEUTIC DIFFERENT DRUGS AFFECTING THE NERVOUS SYSTEM AND TISSUES DRUGS PHARMACOLOGICAL GROUPS Drugs affecting peripheral Antitumor drugs Drugs affecting the cardiovascular Antimicrobial, antiviral, Drugs affecting the nervous system Antiallergic drugs system antiparasitic drugs central nervous system Drugs affecting the sensory Antidotes nerve endings Cardiac glycosides Antibiotics CNS DEPRESSANTS (AFFECTING THE Antihypertensive drugs Sulfonamides Analgesics (opioid, AFFERENT INNERVATION) Antianginal drugs Antituberculous drugs analgesics-antipyretics, Antiarrhythmic drugs Antihelminthic drugs NSAIDs) Local anaesthetics Antihyperlipidemic drugs Antifungal drugs Sedative and hypnotic Coating drugs Spasmolytics Antiviral drugs drugs Adsorbents Drugs affecting the excretory system Antimalarial drugs Tranquilizers Astringents Diuretics Antisyphilitic drugs Neuroleptics Expectorants Drugs affecting the hemopoietic system Antiseptics Anticonvulsants Irritant drugs Drugs affecting blood coagulation Disinfectants Antiparkinsonian drugs Drugs affecting peripheral Drugs affecting erythro- and leukopoiesis General anaesthetics neurotransmitter processes Drugs affecting the digestive system CNS STIMULANTS (AFFECTING THE Anorectic drugs Psychomotor stimulants EFFERENT PART OF THE Bitter stuffs. Drugs for replacement therapy Analeptics NERVOUS SYSTEM) Antiacid drugs Antidepressants Direct-acting-cholinomimetics Antiulcer drugs Nootropics (Cognitive -

Workbook Psychiatry and Narcology
Kharkiv National Medical University Department of Psychiatry, Narcology and Medical Psychology WORKBOOK MANUAL FOR INDIVIDUAL WORK FOR MEDICAL STUDENTS PSYCHIATRY AND NARCOLOGY (Part 2) Student ___________________________________________________________ Faculty _________________________________________________________ Course _________________ Group _____________________________________ Kharkiv 2019 Затверджено вченою радою ХНМУ Протокол №5 від 23.05.2019 р. Psychiatry (Part 2) : workbook manual for individual work of students / I. Strelnikova, G. Samardacova, К. Zelenska – Kharkiv, 2019. – 103 p. Копіювання для розповсюдження в будь-якому вигляді частин або повністю можливо тільки з дозволу авторів навчального посібника. CLASS 7. NEUROTIC DISORDERS. CLINICAL FORMS. TREATMENT AND REHABILITATION. POSTTRAUMATIC STRESS DISORDER. TREATMENT AND REHABILITATION. Psychogenic diseases are a large and clinically varied group of diseases resulting from an effect of acute or long-term psychic traumas, which manifest themselves by both mental and somatoneurological disorders and, as a rule, are reversible. Psychogenic diseases are caused by a psychic trauma, i.e. some events which affect significant aspects of existence of the human being and result in deep psychological feelings. These may be subjectively significant events, i.e. those which are pathogenic for the majority of people. Besides, the psyche may be traumatized by conventionally pathogenic events, which cause feelings in an individual because of his peculiar hierarchy of values. Unfavorable psychogenic effects on the human being cause stress in him, i.e. a nonspecific reaction at the physiological, psychological and behavioural levels. Stress may exert some positive, mobilizing influence, but may result in disorganization of the organism activity. The stress, which exerts a negative influence and causes various disturbances and even diseases, is termed distress. Classification of neurotic disorders I. -

List of Registred Drugs in Armenia (01.03.2017-31.03.2017)
LIST OF REGISTRED DRUGS IN ARMENIA (01.03.2017-31.03.2017) International nonproprietary name Dose and Registration Term of Legal Status N Trade name Drug form Manufacturer Country ATC1 code License holder (generic) or active packaging number registration for Supply ingredients name Eli Lilly Regional Lilly France S.A.S., Operations GmbH., solution for 100IU/ml, Zone Industrielle, 2 17.03.2017 1 Abasaglar insulin glargine France A10AE04 16535 PoM2 Koelblgasse 8-10, injection 3ml cartridges (5) rue du Colonel Lilly, 17.03.2022 1030, Vienna, 67640 Fegersheim Austria Olainfarm JSC, 5 300mg, Olainfarm JSC, 5 06.03.2017 Rupnicu Str., 2 Adaptol mebicar capsules hard in blister (20/2x10/, Rupnicu Str., Olaine, Latvia N06BX21 16440 PoM 06.03.2022 Olaine, LV-2114, 30/3x10/, 40/4x10/) LV-2114 Latvia Olainfarm JSC, 5 Olainfarm JSC, 5 500mg, 06.03.2017 Rupnicu Str., 3 Adaptol mebicar tablets Rupnicu Str., Olaine, Latvia N06BX21 16441 PoM in blister (20/2x10/) 06.03.2022 Olaine, LV-2114, LV-2114 Latvia 10mg, Pharmstandard- OTCPharm PJSC, fabomotizole in blisters Leksredstva JSC, 27.03.2017 123317, Moscow, 4 Afobazol (fabomotizole tablets (30/1x30/, 60/3x20/, 305022, Kursk, Russia N05BX04 14729/2 PoM 30.07.2020 Testovskaya str., 10, dihydrochloride) 60/2x30/, 90/3x30/, Agregatnaya 2nd str., Russia 120/4x30/) 1a/18 Gedeon Richter 15mg/g, Gedeon Richter PLC, 17.03.2017 PLC, Gyomroi ut 5 Airtal aceclofenac cream 60g aluminium Gyomroi ut 19-21, Hungary M01AB16 16514 OTC3 17.03.2022 19-21, 1103 tube 1103 Budapest Budapest, Hungary 1 Gedeon Richter PLC, Gyomroi ut 19-21, 1103 Budapest - batch releaser, Industrias Gedeon Richter Farmaceuticas powder for oral 100mg, 17.03.2017 PLC, Gyomroi ut 6 Airtal aceclofenac Almirall S.L., Ctra. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

LIST of REGISTRED DRUGS in ARMENIA (Up to 31.12.2017)
LIST OF REGISTRED DRUGS IN ARMENIA (Up to 31.12.2017) International nonproprietary Registration Term of Legal status for N Trade name name (generic) or Drug form Dose and packaging Manufacturer Country ATC1 code License holder number registration supply active ingredients name Lek Pharmaceuticals pefloxacin Lek Pharmaceuticals 400mg, 10.03.2015 d.d., Verovskova Str. 1 Abaktal (pefloxacin tablets film-coated d.d., Verovskova Str. Slovenia J01MA03 14308 PoM2 in blister (10/1x10/) 10.03.2020 57, 1526 Ljubljana, mesylate dihydrate) 57, 1526 Ljubljana Slovenia Lilly France S.A.S., Eli Lilly Regional 100IU/ml, Zone Industrielle, 2 17.03.2017 Operations GmbH., 2 Abasaglar insulin glargine solution for injection France A10AE04 16535 PoM 3ml cartridges (5) rue du Colonel Lilly, 17.03.2022 Koelblgasse 8-10, 67640 Fegersheim 1030, Vienna, Austria Help S.A ,10 ambroxol Help S.A. Pedini, Valaoritou str., GR 6mg/ml, 09.03.2016 3 Abrobion (ambroxol syrup Ioanninon, Ioannina, Greece R05CB06 15404 OTC3 144 52, 125ml glass bottle 09.03.2021 hydrochloride) 45500 Metamorphosis, Attika, Greece Salutas Pharma GmbH, Otto-von- Guericke-Alle-1, 100mg/5ml, 39179 Barleben-batch Sandoz 30g powder in 75ml glass powder for oral releaser, Allphamed Pharmaceuticals d.d., bottle and measuring 20.10.2015 4 ACC acetylcysteine solution with orange Pharbil Arzneimittel Germany R05CB01 14947 OTC Verovskova Str. 57, spoon 5ml, 60g powder in 20.10.2020 flavour GmbH Hildebrandstr. 1000 Ljubljana, 150ml glass bottle and 12, 37081 Gottingen, Slovenia measuring spoon 5ml Germany-bulk manufacturer, packager 1 Salutas Pharma GmbH, Otto-von- Guericke-Alle-1, 39179 Barleben - Sandoz batch releaser Pharmaceuticals d.d., 100mg, 21.11.2014 5 ACC 100 acetylcysteine tablets effervescent (Hermes Pharma Germany R05CB01 13967 OTC Verovskova Str. -

WHO Drug Information Vol
WHO Drug Information Vol. 26, No. 4, 2012 WHO Drug Information Contents International Regulatory Regulatory Action and News Harmonization New task force for antibacterial International Conference of Drug drug development 383 Regulatory Authorities 339 NIBSC: new MHRA centre 383 Quality of medicines in a globalized New Pakistan drug regulatory world: focus on active pharma- authority 384 ceutical ingredients. Pre-ICDRA EU clinical trial regulation: public meeting 352 consultation 384 Pegloticase approved for chronic tophaceous gout 385 WHO Programme on Tofacitinib: approved for rheumatoid International Drug Monitoring arthritis 385 Global challenges in medicines Rivaroxaban: extended indication safety 362 approved for blood clotting 385 Omacetaxine mepesuccinate: Safety and Efficacy Issues approved for chronic myelo- Dalfampridine: risk of seizure 371 genous leukaemia 386 Sildenafil: not for pulmonary hyper- Perampanel: approved for partial tension in children 371 onset seizures 386 Interaction: proton pump inhibitors Regorafenib: approved for colorectal and methotrexate 371 cancer 386 Fingolimod: cardiovascular Teriflunomide: approved for multiple monitoring 372 sclerosis 387 Pramipexole: risk of heart failure 372 Ocriplasmin: approved for vitreo- Lyme disease test kits: limitations 373 macular adhesion 387 Anti-androgens: hepatotoxicity 374 Florbetapir 18F: approved for Agomelatine: hepatotoxicity and neuritic plaque density imaging 387 liver failure 375 Insulin degludec: approved for Hypotonic saline in children: fatal diabetes mellitus -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] BMJ Open Pediatric drug utilization in the Western Pacific region: Australia, Japan, South Korea, Hong Kong and Taiwan Journal: BMJ Open ManuscriptFor ID peerbmjopen-2019-032426 review only Article Type: Research Date Submitted by the 27-Jun-2019 Author: Complete List of Authors: Brauer, Ruth; University College London, Research Department of Practice and Policy, School of Pharmacy Wong, Ian; University College London, Research Department of Practice and Policy, School of Pharmacy; University of Hong Kong, Centre for Safe Medication Practice and Research, Department -

Farmaatsia- Terminoloogia Teine, Täiendatud Trükk
Farmaatsia- terminoloogia Teine, täiendatud trükk Graanulid Suspensioon Lahus Emulsioon Pillid Pulber Salv Kreem Aerosool Plaaster Sprei Pastill Tampoon Oblaat Emulsioon Kontsentraat Silmageel Tablett Haavapulk Ninatilgad Kapsel Lakukivi Inhalaator Farmaatsia- terminoloogia Teine, täiendatud trükk Tartu 2019 Koostajad: Toivo Hinrikus, Karin Kogermann, Ott Laius, Signe Leito, Ain Raal, Andres Soosaar, Triin Teppor, Daisy Volmer Keeletoimetaja: Tiina Kuusk Kirjastanud: Ravimiamet Nooruse 1, 50411 Tartu Telefon: +372 737 4140 Faks: +372 737 4142 E-post: [email protected] Esimene trükk 2010 Teine, täiendatud trükk 2019 Raamat on leitav Ravimiameti veebilehelt: www.ravimiamet.ee/farmaatsiaterminoloogia Väljaande refereerimisel või tsiteerimisel palume viidata allikale. ISBN 978-9949-9697-3-9 Sisukord Farmaatsiaterminoloogia Eestis..........................................................................................5 Üldised farmaatsiaalased terminid ...................................................................................10 Euroopa farmakopöa ......................................................................................................... 21 Euroopa farmakopöa sõnastik ..........................................................................................24 Standardterminid ..............................................................................................................29 Ravimvormid .....................................................................................................................29 -
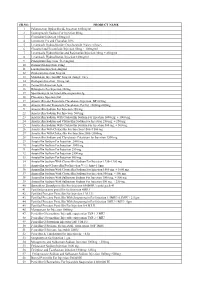
SR.NO. PRODUCT NAME 1 Palonosetron Hydrochloride
SR.NO. PRODUCT NAME 1 Palonosetron Hydrochloride Injection 0.05mg/ml 2 Esomeprazole Sodium For Injection 40mg 3 Cimetidine Injection 100mg/ml 4 Ivermectin 1% and Clorsulon 10% 5 Levamisole Hydrochloride+Oxyclozanide 3%w/v+6%w/v 6 Closantel and Levamisole Injection 50mg + 100mg/ml 7 Levamisole Hydrochloride and Rafoxanide Injection 30mg + 45mg/ml 8 Levamisole Hydrochloride Injection 100mg/ml 9 Praziquantel Injection 56.8 mg/ml 10 Doramectin Injection 10mg 11 Lorazepam Injection 4mg/ml 12 Diazepam Injection 5mg/ml 13 Midazolam Injection BP 5mg/ml (Single Use) 14 Diazepam Injection 10 mg./ml. 15 Temocillin Injection 1gm 16 Rifampicin For Injection 300mg 17 Spectinomycin for Injectable suspension 2g 18 Placentrex Injection 2ml 19 Amoxicillin and Potassium Clavulanate Injection BP 625mg 20 Amoxicillin and Potassium Clavulanate For Inj. 1000mg+200mg 21 Amoxicillin Sodium For Injection 250 mg. 22 Amoxicillin Sodium For Injection 500 mg. 23 Amoxicillin Sodium With Cloxacillin Sodium For Injection 1000 mg. + 1000 mg. 24 Amoxicillin Sodium and Cloxacillin Sodium For Injection 250 mg. + 250 mg. 25 Amoxicillin Sodium With Cloxacillin Sodium For Injection 500 mg. + 500 mg. 26 Amoxicillin With Cloxacillin For Injection 1500+1500 mg 27 Amoxicillin With Cloxacillin For Injection 2000+2000mg 28 Amoxicillin Sodium and Clavulanate Potassium for Injection 1200 mg 29 Ampicillin Sodium For Injection 2000 mg. 30 Ampicillin Sodium For Injection 3000 mg. 31 Ampicillin Sodium For Injection 250 mg. 32 Ampicillin Sodium For Injection 2500 mg. 33 Ampicillin Sodium For Injection 500 mg. 34 Ampicillin Sodium With Cloxacillin Sodium For Injection 1250+1250 mg 35 Ampicillin and Cloxacillin For Injection Vet 1.5gm+1.5gm 36 Ampicillin Sodium With Cloxacillin Sodium For Injection 1000 mg. -

Mexidol® Action
Mexidol has antioxidant, antihypoxic and membrane-protective Mexidol® action. It inhibits lipid peroxidation, increases the activity of superoxide dismutase, increases the lipid-protein ratio, reduces International Non-Proprietary Name (INN): Mexidol the viscosity of the membrane and increases its fluidity. The drug (Emoxypine) modulates the activity of membrane-bound enzymes (calcium of independent phosphodiesterase, adenylate cyclase and Dosage Form: pills (125 mg) acetylcholinesterase) and receptor complexes (benzodiazepine, Structure: 1 capsule contains: GABA and acetylcholine): it enhances their ability to bind to Active ingredient: ethylmethylhydroxypyridine succinate (2-ethyl- ligands, helps to preserve the structural and functional 6-methyl-3-hydroxypyridine) – 125 mg. organization of biomembranes and neurotransmitter transport, Excipients: lactose monohydrate – 97.5 mg, povidone – 25 mg, and facilitates synaptic transmission improvement. magnesium stearate – 2.5 mg. Mexidol causes an increase of dopamine in the brain. It Film coat: opadrai II white 33G28435 – 7.5 mg (hypromellose 3 enhances compensatory activation of aerobic glycolysis and mg, titanium dioxide – 1.875 mg, lactose monohydrate – 1.575 weakens the inhibition of oxidative processes in the Krebs cycle mg, polyethylene glycol (macrogol) – 0.6 mg, triacetin – 0.45 mg) under conditions of hypoxia with an increased ATP and creatine phosphate. The drug activates the energy-synthesizing functions Description: of mitochondria and stabilization of cell membranes. The double radius pills are covered with a film coat in white or Mexidol improves the metabolism and blood supply of the brain, light creamy colour. On the cross-section there are two layers: improves microcirculation and rheological properties of blood, the inner one (the core) is gray or gray-creamy and the outer one and reduces platelet aggregation.