Abuse-Proof Solid Pharmaceutical Composition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Farmaatsia- Terminoloogia Teine, Täiendatud Trükk
Farmaatsia- terminoloogia Teine, täiendatud trükk Graanulid Suspensioon Lahus Emulsioon Pillid Pulber Salv Kreem Aerosool Plaaster Sprei Pastill Tampoon Oblaat Emulsioon Kontsentraat Silmageel Tablett Haavapulk Ninatilgad Kapsel Lakukivi Inhalaator Farmaatsia- terminoloogia Teine, täiendatud trükk Tartu 2019 Koostajad: Toivo Hinrikus, Karin Kogermann, Ott Laius, Signe Leito, Ain Raal, Andres Soosaar, Triin Teppor, Daisy Volmer Keeletoimetaja: Tiina Kuusk Kirjastanud: Ravimiamet Nooruse 1, 50411 Tartu Telefon: +372 737 4140 Faks: +372 737 4142 E-post: [email protected] Esimene trükk 2010 Teine, täiendatud trükk 2019 Raamat on leitav Ravimiameti veebilehelt: www.ravimiamet.ee/farmaatsiaterminoloogia Väljaande refereerimisel või tsiteerimisel palume viidata allikale. ISBN 978-9949-9697-3-9 Sisukord Farmaatsiaterminoloogia Eestis..........................................................................................5 Üldised farmaatsiaalased terminid ...................................................................................10 Euroopa farmakopöa ......................................................................................................... 21 Euroopa farmakopöa sõnastik ..........................................................................................24 Standardterminid ..............................................................................................................29 Ravimvormid .....................................................................................................................29 -
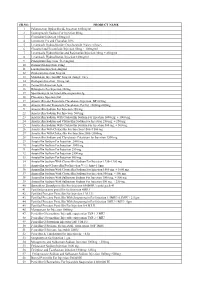
SR.NO. PRODUCT NAME 1 Palonosetron Hydrochloride
SR.NO. PRODUCT NAME 1 Palonosetron Hydrochloride Injection 0.05mg/ml 2 Esomeprazole Sodium For Injection 40mg 3 Cimetidine Injection 100mg/ml 4 Ivermectin 1% and Clorsulon 10% 5 Levamisole Hydrochloride+Oxyclozanide 3%w/v+6%w/v 6 Closantel and Levamisole Injection 50mg + 100mg/ml 7 Levamisole Hydrochloride and Rafoxanide Injection 30mg + 45mg/ml 8 Levamisole Hydrochloride Injection 100mg/ml 9 Praziquantel Injection 56.8 mg/ml 10 Doramectin Injection 10mg 11 Lorazepam Injection 4mg/ml 12 Diazepam Injection 5mg/ml 13 Midazolam Injection BP 5mg/ml (Single Use) 14 Diazepam Injection 10 mg./ml. 15 Temocillin Injection 1gm 16 Rifampicin For Injection 300mg 17 Spectinomycin for Injectable suspension 2g 18 Placentrex Injection 2ml 19 Amoxicillin and Potassium Clavulanate Injection BP 625mg 20 Amoxicillin and Potassium Clavulanate For Inj. 1000mg+200mg 21 Amoxicillin Sodium For Injection 250 mg. 22 Amoxicillin Sodium For Injection 500 mg. 23 Amoxicillin Sodium With Cloxacillin Sodium For Injection 1000 mg. + 1000 mg. 24 Amoxicillin Sodium and Cloxacillin Sodium For Injection 250 mg. + 250 mg. 25 Amoxicillin Sodium With Cloxacillin Sodium For Injection 500 mg. + 500 mg. 26 Amoxicillin With Cloxacillin For Injection 1500+1500 mg 27 Amoxicillin With Cloxacillin For Injection 2000+2000mg 28 Amoxicillin Sodium and Clavulanate Potassium for Injection 1200 mg 29 Ampicillin Sodium For Injection 2000 mg. 30 Ampicillin Sodium For Injection 3000 mg. 31 Ampicillin Sodium For Injection 250 mg. 32 Ampicillin Sodium For Injection 2500 mg. 33 Ampicillin Sodium For Injection 500 mg. 34 Ampicillin Sodium With Cloxacillin Sodium For Injection 1250+1250 mg 35 Ampicillin and Cloxacillin For Injection Vet 1.5gm+1.5gm 36 Ampicillin Sodium With Cloxacillin Sodium For Injection 1000 mg. -

Mexidol® Action
Mexidol has antioxidant, antihypoxic and membrane-protective Mexidol® action. It inhibits lipid peroxidation, increases the activity of superoxide dismutase, increases the lipid-protein ratio, reduces International Non-Proprietary Name (INN): Mexidol the viscosity of the membrane and increases its fluidity. The drug (Emoxypine) modulates the activity of membrane-bound enzymes (calcium of independent phosphodiesterase, adenylate cyclase and Dosage Form: pills (125 mg) acetylcholinesterase) and receptor complexes (benzodiazepine, Structure: 1 capsule contains: GABA and acetylcholine): it enhances their ability to bind to Active ingredient: ethylmethylhydroxypyridine succinate (2-ethyl- ligands, helps to preserve the structural and functional 6-methyl-3-hydroxypyridine) – 125 mg. organization of biomembranes and neurotransmitter transport, Excipients: lactose monohydrate – 97.5 mg, povidone – 25 mg, and facilitates synaptic transmission improvement. magnesium stearate – 2.5 mg. Mexidol causes an increase of dopamine in the brain. It Film coat: opadrai II white 33G28435 – 7.5 mg (hypromellose 3 enhances compensatory activation of aerobic glycolysis and mg, titanium dioxide – 1.875 mg, lactose monohydrate – 1.575 weakens the inhibition of oxidative processes in the Krebs cycle mg, polyethylene glycol (macrogol) – 0.6 mg, triacetin – 0.45 mg) under conditions of hypoxia with an increased ATP and creatine phosphate. The drug activates the energy-synthesizing functions Description: of mitochondria and stabilization of cell membranes. The double radius pills are covered with a film coat in white or Mexidol improves the metabolism and blood supply of the brain, light creamy colour. On the cross-section there are two layers: improves microcirculation and rheological properties of blood, the inner one (the core) is gray or gray-creamy and the outer one and reduces platelet aggregation. -

WO 2017/120012 Al 13 July 20 17 (13.07.2017) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/120012 Al 13 July 20 17 (13.07.2017) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/185 (2006.01) A61K 31/336 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, PCT/US20 16/067024 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KH, KN, 15 December 2016 (15. 12.2016) KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, (25) Filing Language: English NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, (26) Publication Language: English RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, (30) Priority Data: ZA, ZM, ZW. 62/275,182 5 January 2016 (05.01 .2016) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: THE REGENTS OF THE UNIVERSITY kind of regional protection available): ARIPO (BW, GH, OF CALIFORNIA [US/US]; 1111 Franklin Street, 12th GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, Floor, Oakland, California 94607-5200 (US). -

Anapharm Bioanalytics Method List
Bioanalytical Method List SEPTEMBER 2021 Anapharm Bioanalytics Encuny 22, 2nd floor 08038 Barcelona, Spain T. +34 93 223 86 36 [email protected] www.anapharmbioanalytics.com Page 1 / 19 Newly developed or recently updated methods Compound Platform Calibration Range Biological Matrix Canagliflozin UPLC/MS/MS 10-4000 ng/mL Human EDTA Plasma Cholecalciferol (Vitamin D3) LC/MS/MS 0.15-15 ng/mL Human EDTA Plasma Clozapine LC/MS/MS 0.1-50 ng/mL Human EDTA Plasma Dapagliflozin LC/MS/MS 0,5-200 ng/mL Human EDTA Plasma Disulfiram UPLC/MS/MS 0.5-100 ng/mL Human EDTA Plasma Edaravone LC/MS/MS 2-7000 ng/mL Human EDTA Plasma L-Ascorbic Acid LC/MS/MS 100 - 25000 ng/mL Human Lithium Heparinized Plasma Mirabegron UPLC/MS/MS 0.1-100 ng/mL Human EDTA Plasma Olodaterol UPLC/MS/MS 0.2-50 pg/mL Human EDTA Plasma Ramipril; Ramiprilat UPLC/MS/MS 0.2-80 ng/mL; 0.2-80 ng/mL Human EDTA Plasma Riluzole LC/MS/MS 1-1000 ng/mL Human EDTA Plasma Silodosin UPLC/MS/MS 0.2-100 ng/mL Human EDTA Plasma Silodosin Glucuronide UPLC/MS/MS 0.5-200 ng/mL Human EDTA Plasma Temozolomide UPLC/MS/MS 100–25000 ng/mL Human EDTA Plasma Ticagrelor LC/MS/MS 2–1000 ng/mL Human EDTA Plasma Torasemide LC/MS/MS 10-5000 ng/mL Human EDTA Plasma www.anapharmbioanalytics.com Page 2 / 19 List of bioanalytical methods Compound Platform Calibration Range Biological Matrix Abiraterone LC/MS/MS 0.2-100 ng/mL Human EDTA Plasma Abiraterone LC/MS/MS 0.5-200 ng/mL Human EDTA Plasma Aceclofenac LC/MS/MS 15-15000 ng/mL Human EDTA Plasma Acetylsalicylic Acid; Salicylic Acid LC/MS/MS 10-5000 ng/mL; -

An Overview on Non Epileptic Seizures
wjpmr, 2017,3(7), XXX-XXX SJIF Impact Factor: 4.103 WORLD JOURNAL OF PHARMACEUTICAL Research Article ISSN 2455-3301 AND MEDICAL RESEARCH www.wjpmr.com WJPMR wjpmr, 2017,3(7), 185-193 SJIF Impact Factor: 4.103 WORLD JOURNAL OF PHARMACEUTICAL Research Article Aishwarya et al. World Journal of Pharmaceutical and Medical ReISSNsearch 2455 -3301 AND MEDICAL RESEARCH www.wjpmr.com WJPMR AN OVERVIEW ON NON EPILEPTIC SEIZURES M. N. L. Aishwarya*, Prasanna Kumar Kar, P. C. Jayanth and M. Niranajan Babu Department of Pharmacology, Seven Hills College of Pharmacy, Tirupati. *Corresponding Author: M. N. L. Aishwarya Department of Pharmacology, Seven Hills College of Pharmacy, Tirupati. Article Received on 14/06/2017 Article Revised on 05/07/2017 Article Accepted on 26/07/2017 ABSTRACT The brain is susceptible to many different types of disorders that strike at every stage of life. Developmental disorders such as autism and dyslexia first appear in early childhood. Psychiatric diseases such as depression and schizophrenia are typically diagnosed in teens or in early adulthood. Epilepsy is the fourth most common neurological disorder and affects people of all ages. Epilepsy is a group of related disorders characterized by a tendency for recurrent seizures that occur due to abnormal electrical discharges whereas Non Epileptic Seizures are not characterized by abnormal electric discharges in brain. Non Epileptic Seizures also possess greater impact on human life as that of normal epilepsy. This article aims towards discussing Non Epileptic Seizures in detail which includes the classification, epidemiology, types, causes, diagnosis, and treatment of Non Epileptic Seizures including treatment of Pediatric Non Epileptic Seizures. -
Capsules Gastro- Resistant 40Mg, in Blister (7/1X7/, 14/2X7/, 15/3X5/, 28
40mg, KRKA d.d., Novo KRKA d.d., Novo esomeprazole in blister (7/1x7/, 14/2x7/, capsules gastro- mesto, Smarjeska 06.02.2015 mesto, Smarjeska 1554 Emanera (esomeprazole 15/3x5/, 28/4x7/, Slovenia A02BC05 14185 PoM resistant cesta 6, 8501 Novo 06.02.2020 cesta 6, 8501 Novo magnesium) 30/3x10/, 56/8x7/, Mesto Mesto, Slovenia 60/6x10/) Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem - secondary packager, Schering-Plough batch releaser (bulk Central East AG, fosaprepitant powder lyophilized manufacturer, 150mg, 19.08.2016 Weystrasse 20, P.O. 1555 Emend (fosaprepitant for solution for primary packager - the Netherlands A04AD12 12302/3 PoM 10ml glass vial 22.03.2018 Box CH-6000 dimeglumine) infusion Patheon Lucerne 6, Manufacturing Switzerland Services LLC, 5900 Martin Luther King Jr. Higway, Greenville, North Carolina 27834, USA) Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem - secondary packager, Schering-Plough batch releaser (bulk Central East AG, fosaprepitant powder lyophilized manufacturer, 150mg, 08.08.2017 Weystrasse 20, P.O. 1556 Emend (fosaprepitant for solution for primary packager - the Netherlands A04AD12 16883 PoM 10ml glass vial (1) 08.08.2022 Box CH-6000 dimeglumine) infusion Patheon Lucerne 6, Manufacturing Switzerland Services LLC, 5900 Martin Luther King Jr. Higway, Greenville, North Carolina 27834, USA) 341 Belmedpreparaty Belmedpreparaty 10mg/ml, 07.10.2013 RUE (220007, Minsk, 1557 Emoxypine emoxipine drops eye (solution) RUE (220007, Minsk, Belarus S01XA 12895 PoM 5ml glass vial 07.10.2018 Fabritsius -

Belmedpreparaty, RUE Fabriciusa St
Belmedpreparaty, RUE Fabriciusa st. 30, 220007 Minsk, Republic of Belarus Tel/Fax: (+375 17) 217 13 28 [email protected] www.belmedpreparaty.by Dear Sirs, Please accept our sincere regards and respect. «Belmedpreparaty» - the leading pharmaceutical enterprise in the Republic of Belarus, since 1929. The enterprise is the leading one among all the pharmaceutical enterprises in the Republic of Belarus in production capacities and supplying of pharmaceutical products .The enterprise produces 360 sorts of pharmaceutical products of different pharmacological groups. «Belmedpreparaty» has modern high-technology equipment and is the leader in the manufacture of the following pharmaceutical products: - Insulin; - Products for the cancer and tuberculosis treatment; - Narcotic products and Psychotropic substances; - Antibiotics and other has been issuing almost all types of dosage forms: - Tablets with different coating, and without them; - Hard and soft gelatin capsules; - Solutions for injection and infusion in vials and ampoules; - Lyphilisaited and sterile packaged powders for injections and infusions; - Eye drops; - Ointments, creams and gels. The important place of the enterprise’ product list belongs to anticancer products: Fludarobel, Leucladine, Methotrexate, Paclitaxel, Cytarabine, Oxaliplatin, Zoledronic acid and others. Especially the enterprise is proud of the original medicine for photodynamic therapy of oncological diseases - Photolon. Manufacture of pharmaceutical products is certified for compliance with GMP, the enterprise implemented a quality management system in compliance with ISO 9001-2001. «Belmedpreparaty» is supplying products to 14 countries, such as Russia, the Ukraine, Kazakhstan, Azerbaijan, Vietnam, Iraq, USA and others. We are truly interested in developing our export potential, market development and inviting you to collaborate with us in the following directions: 1. -

Substance Abuse in the Workplaces of Our Bodies and Mind
“Turn on, Tune in, Drop out “ Substance Abuse in the Workplaces of our bodies and mind.. Warren Silverman MD Brief History of Drug abuse – Mary Jane 1629 – Marijuana introduced to the Puritan colonies of New England 1765 – George Washington was cultivating Marijuana for a sore tooth 1800’s Tincture of Cannabis is available from pharmacies, unpopular due to variations in potency and dosage, but recreational use continues with Hashish clubs in most cities by 1885 By the 20th century, marijuana use was associated with racial groups and drug abusers and lost popularity The foreign origin of marijuana lead to propaganda against its use (as we have just seen), by 1930’s marijuana was considered wicked In the 1960’s, drug use was considered a demonstration of anti-establishment leanings and became popular Marijuana has remained a constant presence in our society with gradual legislation to decriminalize and legitimize its use Brief History of Drug Abuse - Opiates In colonial America, Opiate medications were common in London and imported to the colonies – used to treat pain from diarrhea, colds, fever, tooth aches, cholera, rheumatism, pelvic disorders, athlete’s foot and baldness 1784 Dr. William Buchan’s book tells people how to make their own tincture of Opium (paregoric) to keep around the house 1804 catalogue listed 90 brands of elixir, by 1905 it was more than 28,000 1803 Morphine developed (Morpheus – god of dreams) Hypodermic needle invented and by the civil war Morphine was widely used as injectable 1898 Heroin developed -
Drugsson Partners Produktlista
DRUGSSON № Product Name Dosage Release form Amount in packing INN (or composition) 1 Acetylcysteine 0.2g powder for oral solution, sachet 20 Acetylcysteine 2 Acetylcysteine 20% 5ml solution for inhalation 10 Acetylcysteine 3 Acetylsalicylic acid 500mg tablets 20 Acetylsalicylic acid 4 Acetylsalicylic acid 50mg lyophilized powder for solution for injection 1 Acetylsalicylic acid 5 Acidin-Pepsin tablets 50 Betain + Pepsin 6 Acyclovir 250mg freeze-dried powder for injection, vial 1 Acyclovir 7 Acyclovir 500mg freeze-dried powder for injection, vial 1 Acyclovir 8 Acyclovir 1000mg freeze-dried powder for injection, vial 1 Acyclovir 9 Acyclovir 50mg/g 5g ointment 1 Acyclovir 10 Acyclovir 200 mg tablets 20 Acyclovir 11 Aevit capsules 50 Vitamin A and E 12 Aktovir 4g ointment 1 Acyclovir + Butaminophene 13 Alendronic acid 70mg tablets 4 Alendronic acid Bile + Garlic + Nettle leaves 14 Allochol coated tablets 50 + Activated Carbon 15 Alpha-tocopherol acetate 100mg capsules 30 Vitamin E Ferric Chloride Hexahydrate, 16 Alustat 10ml solution for external use, vial 1 Aluminium Chloride Hexahydrate Amoxicillin + beta-lactamase 17 Amklav 500 mg+100 mg powder for solution for intravenous use, vial 1 inhibitor Amoxicillin + beta-lactamase 18 Amklav 1000 mg+200 mg powder for solution for intravenous use, vial 1 inhibitor Amoxicillin + beta-lactamase 19 Amklav 250 mg+125 mg coated tablets, vial 15 inhibitor 20 Amlodipine 5mg tablets 30 Amlodipine 21 Amoxicillin 250mg capsules 30 Amoxicillin 22 Ampicillin trihydrate 250mg tablets 20 Ampicillin trihydrate 23 Analgin 500mg tablets 20 Metamizole sodium 24 Anastrozole 1mg coated tablets 30 Anastrozole 25 Artificial tear 1ml eye drops, dropping tubes 2 Hypromellose + Dextran 26 Ascorbic acid 25 mg tablets 20 Ascorbic acid 27 Ascorbic acid with glucose 100 mg/ 877 mg tablets 20 Ascorbic acid + Glucose Potassium aspartate. -

244 Original Article: Features of Leukocytes' Apoptosis And
Bangladesh Journal of Medical Science Vol. 18 No. 02 April’19 Original article: Features of leukocytes’ apoptosis and emoxypine succinate efficacy in case of combined trauma of the chest and both thighs in rats Inna Krynytska1, Mariya Marushchak2, Liudmyla Holovatiuk3, Leonid Shkrobot4, Natalia Sokhor5, Julia Stepas6 Abstract: Objective: This study aims to establish features of blood leukocytes’ apoptosis and substantiate the efficacy of emoxypine succinate applying in case of combined trauma of the chest and both thighs in rats. Materials and Methods: Analysis of cell samples to determine reactive oxygen species was evaluated by the flow laser cytometry method, using 2.7-dichlorodihydrofluorescein diacetate (Sigma Aldrich, Germany). The number of leukocytes with low mitochondrial transmembrane potential was evaluated by the flow laser cytometry method, using a kit of reagents «MitoScreen» («BD Pharmingen», USA). The number of apoptotic leukocytes was evaluated by the flow laser cytometry method, using a kit of reagents “ANNEXIN V FITC” (“Beckman Coulter”, USA). Emoxypine succinate to animals was injected intraperitoneally 1 time per day during 14 days from the first day of experiment in the dosage of 40 mg/kg. Results and Discussion: It was established the progressive, statistically significant increasing of Annexin V- positive cells percentage from the first day of the combined trauma of the chest and both thighs in rats with the highest values within 7-14 days of observation. On 28 day of experiment the reduction of apoptotic white blood cells percentage by 7.7% than the findings on 14 day was observed, but it remained 33.3% higher than control. The analysis of data in case of emoxypine succinate applying indicates that production of reactive oxygen species by leukocytes began to decline after 3 days of experiment and continued to decrease with maximum of action on 7 day. -

Anxiolytic and Antidepressant Actions of Emoxypine, Reamberin, and Mexidol in Experimental Diabetes Mellitus
DOI 10.1007/s11055-018-0706-1 Neuroscience and Behavioral Physiology, Vol. 49, No. 1, January, 2019 Anxiolytic and Antidepressant Actions of Emoxypine, Reamberin, and Mexidol in Experimental Diabetes Mellitus I. A. Volchegorskii, I. Yu. Miroshnichenko, L. M. Rassokhina, R. M. Faizullin, and K. E. Pryakhina Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 117, No. 5, Iss. 1, pp. 52–57, May, 2017. Objectives. To conduct a comparative study of the anxiolytic and antidepressant activities of derivatives of 3-hydroxypyridine and succinic acid (emoxypine, Reamberin, and Mexidol) in experimental diabetes mellitus (DM). Materials and methods. The effects of emoxypine, Reamberin, and Mexidol on the signs of anxiety in an elevated plus maze and the duration of “despair” behavior in the Porsolt test were assessed in rats with alloxan diabetes during courses of drug treatment. The reference agent was α-lipoic acid. An additional series of experiments was run to study the effects of emoxypine, Reamberin, Mexidol and α-li- poic acid on the severity of hyperglycemia in experimental DM. Results and conclusions. All study drugs were used at doses equivalent to the human therapeutic range for 14 days and signifi cantly decreased the signs of anxiety and depression in rats with alloxan diabetes. The most marked anxiolytic potential was demonstrated for emoxypine, which was the only one of the study drugs decreasing the signs of anxiety not only in comparison with the alloxan diabetes control group, but also relative to the intact control group. Derivatives of 3-hydroxypyridine and succinic acid were no less effective than α-lipoic acid in terms of the level of tranquilizing activity and were more effective than α-lipoic acid in terms of thymoanaleptic activity when given at the maximal dose to rats with experimental DM.