Anapharm Bioanalytics Method List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abuse-Proof Solid Pharmaceutical Composition
(19) TZZ¥¥ ¥_T (11) EP 3 329 939 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 06.06.2018 Bulletin 2018/23 A61K 47/02 (2006.01) A61K 47/36 (2006.01) A61K 9/16 (2006.01) A61K 31/485 (2006.01) (2006.01) (21) Application number: 17204525.4 A61K 31/192 (22) Date of filing: 29.11.2017 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • BOSCHETTI, Silvia GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO I-38060 Aldeno (TN) (IT) PL PT RO RS SE SI SK SM TR • ROSSI, Massimiliano Designated Extension States: I-38121 Trento (IT) BA ME • BENFENATI, Diego Designated Validation States: I-38052 Caldonazzo (TN) (IT) MA MD • POJER, Alessandro I-38092 Altavalle (TN) (IT) (30) Priority: 02.12.2016 IT 201600122469 (74) Representative: Allaix, Roberto (71) Applicant: E-Pharma Trento S.p.A. PGA S.p.A. 38123 Trento (IT) Via Mascheroni, 31 20145 Milano (IT) (54) ABUSE-PROOF SOLID PHARMACEUTICAL COMPOSITION (57) The present invention relates to an abuse-proof solid pharmaceutical composition comprising an active ingre- dient with potential for abuse, silica in an amount of from 10 mg to 1000 mg, guar flour in an amount of from 100 mg to 300 mg, and a water soluble diluent in an amount of from 500 mg to 5000 mg. EP 3 329 939 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 329 939 A1 Description FIELD OF THE INVENTION 5 [0001] The present invention relates to an abuse-proof solid pharmaceutical composition, in particular a solid phar- maceutical composition, such as, for example a granulate or a tablet, capable of solubilizing in a glass of water (about 100 ml), but of forming a not injectable gel and/or viscous solution if added to the amount of water contained in a hypodermic syringe (about 10 ml). -

Screening of Pharmaceuticals in San Francisco Bay Wastewater
Screening of Pharmaceuticals in San Francisco Bay Wastewater Prepared by Diana Lin Rebecca Sutton Jennifer Sun John Ross San Francisco Estuary Institute CONTRIBUTION NO. 910 / October 2018 Pharmaceuticals in Wastewater Technical Report Executive Summary Previous studies have shown that pharmaceuticals are widely detected in San Francisco Bay, and some compounds occasionally approach levels of concern for wildlife. In 2016 and 2017, seven wastewater treatment facilities located throughout the Bay Area voluntarily collected wastewater samples and funded analyses for 104 pharmaceutical compounds. This dataset represents the most comprehensive analysis of pharmaceuticals in wastewater to date in this region. On behalf of the Regional Monitoring Program for Water Quality in San Francisco Bay (RMP), the complete dataset was reviewed utilizing RMP quality assurance methods. An analysis of influent and effluent information is summarized in this report, and is intended to inform future monitoring recommendations for the Bay. Influent and effluent concentration ranges measured were generally within the same order of magnitude as other US studies, with a few exceptions for effluent. Effluent concentrations were generally significantly lower than influent concentrations, though estimated removal efficiency varied by pharmaceutical, and in some cases, by treatment type. These removal efficiencies were generally consistent with those reported in other studies in the US. Pharmaceuticals detected at the highest concentrations and with the highest frequencies in effluent were commonly used drugs, including treatments for diabetes and high blood pressure, antibiotics, diuretics, and anticonvulsants. For pharmaceuticals detected in discharged effluent, screening exercises were conducted to determine which might be appropriate candidates for further examination and potential monitoring in the Bay. -

Ovid MEDLINE(R)
Supplementary material BMJ Open Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily <1946 to September 16, 2019> # Searches Results 1 exp Hypertension/ 247434 2 hypertens*.tw,kf. 420857 3 ((high* or elevat* or greater* or control*) adj4 (blood or systolic or diastolic) adj4 68657 pressure*).tw,kf. 4 1 or 2 or 3 501365 5 Sex Characteristics/ 52287 6 Sex/ 7632 7 Sex ratio/ 9049 8 Sex Factors/ 254781 9 ((sex* or gender* or man or men or male* or woman or women or female*) adj3 336361 (difference* or different or characteristic* or ratio* or factor* or imbalanc* or issue* or specific* or disparit* or dependen* or dimorphism* or gap or gaps or influenc* or discrepan* or distribut* or composition*)).tw,kf. 10 or/5-9 559186 11 4 and 10 24653 12 exp Antihypertensive Agents/ 254343 13 (antihypertensiv* or anti-hypertensiv* or ((anti?hyperten* or anti-hyperten*) adj5 52111 (therap* or treat* or effective*))).tw,kf. 14 Calcium Channel Blockers/ 36287 15 (calcium adj2 (channel* or exogenous*) adj2 (block* or inhibitor* or 20534 antagonist*)).tw,kf. 16 (agatoxin or amlodipine or anipamil or aranidipine or atagabalin or azelnidipine or 86627 azidodiltiazem or azidopamil or azidopine or belfosdil or benidipine or bepridil or brinazarone or calciseptine or caroverine or cilnidipine or clentiazem or clevidipine or columbianadin or conotoxin or cronidipine or darodipine or deacetyl n nordiltiazem or deacetyl n o dinordiltiazem or deacetyl o nordiltiazem or deacetyldiltiazem or dealkylnorverapamil or dealkylverapamil -

(12) United States Patent (10) Patent No.: US 6,487,446 B1 Hill Et Al
USOO6487446B1 (12) United States Patent (10) Patent No.: US 6,487,446 B1 Hill et al. (45) Date of Patent: Nov. 26, 2002 (54) METHOD AND SYSTEM FOR SPINAL CORD WO 92/11064 7/1992 STIMULATION PRIOR TO AND DURING A WO 97/40885 11/1997 MEDICAL PROCEDURE WO WO 99/09971 8/1998 WO WO 99/09973 8/1998 (75) Inventors: Michael R.S. Hill, Minneapolis, MN WO 99/07354 2/1999 (US); Scott E. Jahns, Hudson, WI WO O1/OO273 1/2001 (US); James R. Keogh, Maplewood, OTHER PUBLICATIONS MN (US) US 6,184,239, 2/2001, Puskas (withdrawn) (73) Assignee: Medtronic, Inc., Minneapolis, MN An article entitled “Coronary artery Surgery with induced (US) temporary asyStole and intermittent ventricular pacing: an experimental study” by R. Khanna and H.C. Cullen, dated (*) Notice: Subject to any disclaimer, the term of this Apr. 1996, taken from Cardiovascular Surgery, vol. 4, No. patent is extended or adjusted under 35 2, pp. 231-236. U.S.C. 154(b) by 115 days. An unnamed editorial by Adrian R. M. Upton, dated Oct. 1992, taken from PACE vol. 15, pp. 1543–1544. (21) Appl. No.: 09/669,960 An article entitled “Selective Stimulation of Parasympa 1-1. thetic Nerve Fibers to the Human Sinoatrial Node,” by Mark (22) Filed: Sep. 26, 2000 D. Carlson, Alexander S. Geha, Jack Hsu, Paul J. Martin, (51) Int. Cl. ............................. A61N 1/30. A61N 1/18 Matthew N. Levy, Gretta Jacobs and Albert J. Waldo, dated (52) U.S. Cl. .............................. 60420 607/9, 607/117 Apr. 1992, taken from Circulation vol. -

Formulation Development Strategies
FORMULATION DEVELOPMENT STRATEGIES FOR ORAL EXTENDED RELEASE DOSAGE FORMS Dissertation zur Erlangung des akademischen Grades des Doktors der Naturwissenschaften (Dr. rer. nat.) eingereicht im Fachbereich Biologie, Chemie, Pharmazie der Freien Universität Berlin vorgelegt von ARAYA RAIWA aus Bangkok, Thailand May, 2011 1. Gutachter: Prof. Dr. Roland Bodmeier 2. Gutachter: Prof. Dr. Jürgen Siepmann Disputation am 9.Juni 2011 TO MY FAMILY ACKNOWLEDGEMENTS First and foremost, I wish to express my deepest gratitude to my supervisor, Prof. Dr. Roland Bodmeier for his professional guidance, helpful advices and encouragement. I am very grateful for his scientific and financial support and for providing me such an interesting topic. Furthermore, I am very thankful to him for the opportunity to support his editorial role in the European Journal of Pharmaceutical Sciences. I would like to thank Prof. Dr. Jürgen Siepmann for co-evaluating this thesis. Thanks are extended to Prof. Dr. Herbert Kolodziej, Prof. Dr. Johannes Peter Surmann and Dr. Martin Körber for serving as members of my thesis advisor committee. I am particular thankful to Dr. Andrei Dashevsky, Dr. Nantharat Pearnchob and Dr. Martin Körber for their very useful discussion; Dr. Burkhard Dickenhorst for evaluating parts of this thesis; Mrs. Angelika Schwarz for her assistance with administrative issues; Mr. Andreas Krause, Mrs. Eva Ewest and Mr. Stefan Walter for the prompt and diligent technical support. Sincere thanks are extended to Dr. Ildiko Terebesi, Dr. Burkhard Dickenhorst, Dr. Soravoot Rujivipat and Dr. Samar El-Samaligy for the friendly atmosphere in the lab My special thanks are owing to all members from the Kelchstrasse for their practical advice, enjoyable discussion and kindness throughout the years. -

(12) United States Patent (10) Patent No.: US 9,283,192 B2 Mullen Et Al
US009283192B2 (12) United States Patent (10) Patent No.: US 9,283,192 B2 Mullen et al. (45) Date of Patent: Mar. 15, 2016 (54) DELAYED PROLONGED DRUG DELIVERY 2009. O1553.58 A1 6/2009 Diaz et al. 2009,02976O1 A1 12/2009 Vergnault et al. 2010.0040557 A1 2/2010 Keet al. (75) Inventors: Alexander Mullen, Glasgow (GB); 2013, OO17262 A1 1/2013 Mullen et al. Howard Stevens, Glasgow (GB); Sarah 2013/0022676 A1 1/2013 Mullen et al. Eccleston, Scotstoun (GB) FOREIGN PATENT DOCUMENTS (73) Assignee: UNIVERSITY OF STRATHCLYDE, Glasgow (GB) EP O 546593 A1 6, 1993 EP 1064937 1, 2001 EP 1607 O92 A1 12/2005 (*) Notice: Subject to any disclaimer, the term of this EP 2098 250 A1 9, 2009 patent is extended or adjusted under 35 JP HO5-194188 A 8, 1993 U.S.C. 154(b) by 0 days. JP 2001-515854. A 9, 2001 JP 2001-322927 A 11, 2001 JP 2003-503340 A 1, 2003 (21) Appl. No.: 131582,926 JP 2004-300148 A 10, 2004 JP 2005-508326 A 3, 2005 (22) PCT Filed: Mar. 4, 2011 JP 2005-508327 A 3, 2005 JP 2005-508328 A 3, 2005 (86). PCT No.: PCT/GB2O11AOOO3O7 JP 2005-510477 A 4/2005 JP 2008-517970 A 5, 2008 JP 2009-514989 4/2009 S371 (c)(1), WO WO99,12524 A1 3, 1999 (2), (4) Date: Oct. 2, 2012 WO WOO1 OO181 A2 1, 2001 WO WOO3,O266.15 A2 4/2003 (87) PCT Pub. No.: WO2011/107750 WO WOO3,O26625 A1 4/2003 WO WO 03/026626 A2 4/2003 PCT Pub. -

Farmaatsia- Terminoloogia Teine, Täiendatud Trükk
Farmaatsia- terminoloogia Teine, täiendatud trükk Graanulid Suspensioon Lahus Emulsioon Pillid Pulber Salv Kreem Aerosool Plaaster Sprei Pastill Tampoon Oblaat Emulsioon Kontsentraat Silmageel Tablett Haavapulk Ninatilgad Kapsel Lakukivi Inhalaator Farmaatsia- terminoloogia Teine, täiendatud trükk Tartu 2019 Koostajad: Toivo Hinrikus, Karin Kogermann, Ott Laius, Signe Leito, Ain Raal, Andres Soosaar, Triin Teppor, Daisy Volmer Keeletoimetaja: Tiina Kuusk Kirjastanud: Ravimiamet Nooruse 1, 50411 Tartu Telefon: +372 737 4140 Faks: +372 737 4142 E-post: [email protected] Esimene trükk 2010 Teine, täiendatud trükk 2019 Raamat on leitav Ravimiameti veebilehelt: www.ravimiamet.ee/farmaatsiaterminoloogia Väljaande refereerimisel või tsiteerimisel palume viidata allikale. ISBN 978-9949-9697-3-9 Sisukord Farmaatsiaterminoloogia Eestis..........................................................................................5 Üldised farmaatsiaalased terminid ...................................................................................10 Euroopa farmakopöa ......................................................................................................... 21 Euroopa farmakopöa sõnastik ..........................................................................................24 Standardterminid ..............................................................................................................29 Ravimvormid .....................................................................................................................29 -

Signed Dissertation Title Page Debi Hudgens
Di- and Mono- Phenyl Amine Based Sodium Channel Blockers for the Treatment of Pain Debjani Patangia Hudgens Little Rock, AR B.S., University of Arkansas, 2000 A Dissertation presented to the Graduate Faculty of the University of Virginia in Candidacy for the Degree of Doctor of Philosophy Department of Chemistry University of Virginia January, 2007 Acknowledgements I would like to thank the collaborative efforts of several groups who have made this body of work possible. Chapters 2 & 3 All of the [3H]-BTX and [3H]-norepinephrine binding assays were conducted by Novascreen Biosciences, Inc. A great deal of thanks goes the laboratory of Dr. Manoj K. Patel, particularly Catherine Taylor and Dr. Timothy Batts, for the electrophysiological work conducted on sodium channels. Merck, Division of Ion Channel Research, carried out all of the fluorescence based assay work in collaborative efforts with the Brown lab. The NIH screening program at NINDS was responsible for anticonvulsant and toxicity screening in animal models. I would also like to thank the work of Dr. Steve White’s lab, particularly Misty Smith-Yockman, whose efforts in conjunction with NIH has provided for screening in animal models of inflammatory pain. Chapter 5 I would like to thank my advisor, Dr. Milton Brown, for help with the CoMFA model and prediction of future analogues. All of the [3H]-BTX binding assays were conducted by Novascreen Biosciences, Inc. Electrophysiological screening in calcium channels was conducted in the laboratory of Dr. Yong Kim, with special thanks to Nathan Lewis and Maiko Sakai. Chapter 6 I would also like to thank Dr. -

TARKA® (Trandolapril/Verapamil Hydrochloride ER Tablets) WARNING
TARKA® (trandolapril/verapamil hydrochloride ER tablets) WARNING: FETAL TOXICITY • When pregnancy is detected, discontinue TARKA as soon as possible. • Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus (see WARNINGS: Fetal Toxicity). DESCRIPTION TARKA (trandolapril/verapamil hydrochloride ER) combines a slow release formulation of a calcium channel blocker, verapamil hydrochloride, and an immediate release formulation of an angiotensin converting enzyme inhibitor, trandolapril. Verapamil Component Verapamil hydrochloride is chemically described as benzeneacetonitrile, α[3-[[2-(3,4 dimethoxyphenyl)ethyl]methylamino]propyl]-3, 4-dimethoxy-α-(1-methylethyl) hydrochloride. Its empirical formula is C27H38N2O4 HCl and its structural formula is: Verapamil hydrochloride is an almost white crystalline powder, with a molecular weight of 491.08. It is soluble in water, chloroform, and methanol. It is practically free of odor, with a bitter taste. Trandolapril Component Trandolapril is the ethyl ester prodrug of a nonsulfhydryl angiotensin converting enzyme (ACE) inhibitor, trandolaprilat. It is chemically described as (2S,3aR,7aS)-1-[(S)-N-[(S)-1-Carboxy-3 phenylpropyl]alanyl] hexahydro-2-indolinecarboxylic acid, 1-ethyl ester. Its empirical formula is C24 H34 N2O5 and its structural formula is: Reference ID: 4480017 Trandolapril is a white or almost white powder with a molecular weight of 430.54. It is soluble (>100 mg/mL) in chloroform, dichloromethane, and methanol. TARKA tablets are formulated for oral administration, containing verapamil hydrochloride as a controlled release formulation and trandolapril as an immediate release formulation. The tablet strengths are trandolapril 2 mg/verapamil hydrochloride ER 180 mg, trandolapril 1 mg/verapamil hydrochloride ER 240 mg, trandolapril 2 mg/verapamil hydrochloride ER 240 mg, and trandolapril 4 mg/verapamil hydrochloride ER 240 mg. -

In Partial- Fu1fílnent of the Requirenents for the Degree Of
EFFECT8 OF CHRONIC VERÀPÀ¡,ITIJ ÃDMINISTRÀTION ON EIIE BIOCHE¡,ÍICAL CIIÀRACTERISTICS OF TTÍE IJ-TYPE CAIJCIUM CHÄNNEI.¡ BY BIJAIR BURTON IJONSBERRY A Thesis Subnitted to the Facul-ty of Graduate Studies in Partial- Fu1fílnent of the Requirenents for the Degree of Masters of science Departnent of Physiol-ogy Faculty of Medicine University of Manitoba Winnipeg, Manitoba Septenber, 1993 Bibl¡othèque nationale K*& |,*lå!';o^o du Canada Acquisitions and Direction des acquisitions et Bibliographic Services Branch des services bibliographiques 395 Wellinoton St¡eet 395, rue Wellinglon onawa, oñtario Onawa (Onlarjo) KlA ON4 K1A ON4 Out l¡le No¡rc ftlè¡e.ce The author has granted an L'auteur a accordé une licence írrevocable non-exclusive licence irrévocable et non exclusive allowing the National Library of permettant à la Bibliothèque Canada to reproduce, loan, nationale du Canada de distribute or sell copíes of reproduire, prêter, distribuer ou his/her thesis by any means and vendre des copies de sa thèse in any form or format, making de quelque manière et sous this thesis available to interested quelque forme que ce soit pour persons. mettre des exemplaires de cette thèse à la disposition des personnes intéressées. The author retains ownership of L'auteur conserve la propriété du the copyright in his/her thesís. droit d'auteur qui protège sa Neither the thesis nor substantial thèse. NÍ la thèse ni des extraits extracts from it may be printed or substantiels de celle.ci ne otherwise reproduced without doivent être imprimés ou his/her permission. autrement reproduits sans son autorisation. ISBN 0-315-85935-0 C¿nadä Nt^-- Disserlolion Abstrqcls lnlernolionolis orronged by brood, g{nerol subiect cotegories. -
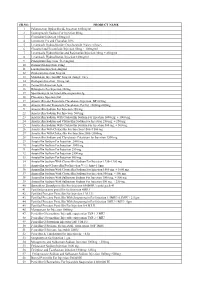
SR.NO. PRODUCT NAME 1 Palonosetron Hydrochloride
SR.NO. PRODUCT NAME 1 Palonosetron Hydrochloride Injection 0.05mg/ml 2 Esomeprazole Sodium For Injection 40mg 3 Cimetidine Injection 100mg/ml 4 Ivermectin 1% and Clorsulon 10% 5 Levamisole Hydrochloride+Oxyclozanide 3%w/v+6%w/v 6 Closantel and Levamisole Injection 50mg + 100mg/ml 7 Levamisole Hydrochloride and Rafoxanide Injection 30mg + 45mg/ml 8 Levamisole Hydrochloride Injection 100mg/ml 9 Praziquantel Injection 56.8 mg/ml 10 Doramectin Injection 10mg 11 Lorazepam Injection 4mg/ml 12 Diazepam Injection 5mg/ml 13 Midazolam Injection BP 5mg/ml (Single Use) 14 Diazepam Injection 10 mg./ml. 15 Temocillin Injection 1gm 16 Rifampicin For Injection 300mg 17 Spectinomycin for Injectable suspension 2g 18 Placentrex Injection 2ml 19 Amoxicillin and Potassium Clavulanate Injection BP 625mg 20 Amoxicillin and Potassium Clavulanate For Inj. 1000mg+200mg 21 Amoxicillin Sodium For Injection 250 mg. 22 Amoxicillin Sodium For Injection 500 mg. 23 Amoxicillin Sodium With Cloxacillin Sodium For Injection 1000 mg. + 1000 mg. 24 Amoxicillin Sodium and Cloxacillin Sodium For Injection 250 mg. + 250 mg. 25 Amoxicillin Sodium With Cloxacillin Sodium For Injection 500 mg. + 500 mg. 26 Amoxicillin With Cloxacillin For Injection 1500+1500 mg 27 Amoxicillin With Cloxacillin For Injection 2000+2000mg 28 Amoxicillin Sodium and Clavulanate Potassium for Injection 1200 mg 29 Ampicillin Sodium For Injection 2000 mg. 30 Ampicillin Sodium For Injection 3000 mg. 31 Ampicillin Sodium For Injection 250 mg. 32 Ampicillin Sodium For Injection 2500 mg. 33 Ampicillin Sodium For Injection 500 mg. 34 Ampicillin Sodium With Cloxacillin Sodium For Injection 1250+1250 mg 35 Ampicillin and Cloxacillin For Injection Vet 1.5gm+1.5gm 36 Ampicillin Sodium With Cloxacillin Sodium For Injection 1000 mg. -

Mexidol® Action
Mexidol has antioxidant, antihypoxic and membrane-protective Mexidol® action. It inhibits lipid peroxidation, increases the activity of superoxide dismutase, increases the lipid-protein ratio, reduces International Non-Proprietary Name (INN): Mexidol the viscosity of the membrane and increases its fluidity. The drug (Emoxypine) modulates the activity of membrane-bound enzymes (calcium of independent phosphodiesterase, adenylate cyclase and Dosage Form: pills (125 mg) acetylcholinesterase) and receptor complexes (benzodiazepine, Structure: 1 capsule contains: GABA and acetylcholine): it enhances their ability to bind to Active ingredient: ethylmethylhydroxypyridine succinate (2-ethyl- ligands, helps to preserve the structural and functional 6-methyl-3-hydroxypyridine) – 125 mg. organization of biomembranes and neurotransmitter transport, Excipients: lactose monohydrate – 97.5 mg, povidone – 25 mg, and facilitates synaptic transmission improvement. magnesium stearate – 2.5 mg. Mexidol causes an increase of dopamine in the brain. It Film coat: opadrai II white 33G28435 – 7.5 mg (hypromellose 3 enhances compensatory activation of aerobic glycolysis and mg, titanium dioxide – 1.875 mg, lactose monohydrate – 1.575 weakens the inhibition of oxidative processes in the Krebs cycle mg, polyethylene glycol (macrogol) – 0.6 mg, triacetin – 0.45 mg) under conditions of hypoxia with an increased ATP and creatine phosphate. The drug activates the energy-synthesizing functions Description: of mitochondria and stabilization of cell membranes. The double radius pills are covered with a film coat in white or Mexidol improves the metabolism and blood supply of the brain, light creamy colour. On the cross-section there are two layers: improves microcirculation and rheological properties of blood, the inner one (the core) is gray or gray-creamy and the outer one and reduces platelet aggregation.