Study on the Population Structure of the Paradoxical Frog, Pseudis Bolbodactyla (Amphibia: Anura: Hylidae), Using Natural Markings for Individual Identification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mudança Climática, Configuração Da Paisagem E Seus Efeitos Sobre a Fenologia E Biodiversidade De Anuros
i INSTITUTO FEDERAL DE EDUCAÇÃO, CIÊNCIA E TECNOLOGIA GOIANO - CAMPUS RIO VERDE PROGRAMA DE PÓS-GRADUAÇÃO BIODIVERSIDADE E CONSERVAÇÃO MUDANÇA CLIMÁTICA, CONFIGURAÇÃO DA PAISAGEM E SEUS EFEITOS SOBRE A FENOLOGIA E BIODIVERSIDADE DE ANUROS Autor: Seixas Rezende Oliveira Orientador: Dr. Matheus de Souza Lima Ribeiro Coorientador: Dr. Alessandro Ribeiro de Morais RIO VERDE – GO Fevereiro – 2018 ii INSTITUTO FEDERAL DE EDUCAÇÃO, CIÊNCIA E TECNOLOGIA GOIANO - CAMPUS RIO VERDE PROGRAMA DE PÓS- GRADUAÇÃO BIODIVERSIDADE E CONSERVAÇÃO MUDANÇA CLIMÁTICA, CONFIGURAÇÃO DA PAISAGEM E SEUS EFEITOS SOBRE A FENOLOGIA E BIODIVERSIDADE DE ANUROS Autor: Seixas Rezende Oliveira Orientador: Dr. Matheus de Souza Lima Ribeiro Coorientador: Dr. Alessandro Ribeiro de Morais Dissertação apresentada, como parte das exigências para obtenção do título de MESTRE EM BIODIVERSIDADE E CONSERVAÇÃO, no Programa de Pós- Graduação em Biodiversidade e conservação do Instituto Federal de Educação, Ciência e Tecnologia Goiano – Campus Rio Verde - Área de Concentração Conservação dos recursos naturais. RIO VERDE – GO Fevereiro – 2018 iii iv v DEDICO ESTE TRABALHO: Aos meus amados pais João Batista Oliveira Rezende e Rita Maria Rezende Oliveira. À meu irmão Fagner Rezende Oliveira e a meus sobrinhos Jorge Otavio Rezende Valdez e João Miguel Rezende Valdez. vi AGRADECIMENTOS A toda minha família, em especial Pai, Mãe e Irmão que nunca mediram esforços para que eu seguisse firme nos estudos, e proporcionaram a mim educação, um lar confortante e seguro, onde sempre busquei minhas forças e inspirações para seguir em frente com todos os projetos de vida. Ao meu orientador e amigo Prof. Dr. Matheus de Souza Lima Ribeiro, exemplo de pessoa em todos os quesitos, falta adjetivos que descreve tamanhas qualidades, que mesmo com muitos afazeres, sempre doou seu tempo para me ajudar sendo essencial para elaboração e condução deste trabalho. -

The Mitochondrial Genome of the Endemic Brazilian Paradoxial Frog Pseudis Tocantins (Hylidae)
University of Kentucky UKnowledge Biology Faculty Publications Biology 10-26-2018 The itM ochondrial Genome of the Endemic Brazilian Paradoxial Frog Pseudis tocantins (Hylidae) Kaleb Pretto Gatto University of Campinas, Brazil Jeramiah J. Smith University of Kentucky, [email protected] Luciana Bolsoni Lourenço University of Campinas, Brazil Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/biology_facpub Part of the Biology Commons, and the Genetics and Genomics Commons Repository Citation Gatto, Kaleb Pretto; Smith, Jeramiah J.; and Lourenço, Luciana Bolsoni, "The itM ochondrial Genome of the Endemic Brazilian Paradoxial Frog Pseudis tocantins (Hylidae)" (2018). Biology Faculty Publications. 160. https://uknowledge.uky.edu/biology_facpub/160 This Article is brought to you for free and open access by the Biology at UKnowledge. It has been accepted for inclusion in Biology Faculty Publications by an authorized administrator of UKnowledge. For more information, please contact [email protected]. The Mitochondrial Genome of the Endemic Brazilian Paradoxial Frog Pseudis tocantins (Hylidae) Notes/Citation Information Published in Mitochondrial DNA Part B, v. 3, no. 2, p. 1106-1107. © 2018 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Digital Object Identifier (DOI) https://doi.org/10.1080/23802359.2018.1508385 This article is available at UKnowledge: https://uknowledge.uky.edu/biology_facpub/160 Mitochondrial DNA Part B Resources ISSN: (Print) 2380-2359 (Online) Journal homepage: https://www.tandfonline.com/loi/tmdn20 The mitochondrial genome of the endemic Brazilian paradoxical frog Pseudis tocantins (Hylidae) Kaleb Pretto Gatto, Jeramiah J. -

Histomorfología De La Glándula Tiroides Durante La Ontogenia En Pseudis Paradoxa (Anura, Hylidae)
Tesis Doctoral Histomorfología de la glándula tiroides durante la ontogenia en Pseudis paradoxa (Anura, Hylidae) Cruz, Julio César 2017 Este documento forma parte de las colecciones digitales de la Biblioteca Central Dr. Luis Federico Leloir, disponible en bibliotecadigital.exactas.uba.ar. Su utilización debe ser acompañada por la cita bibliográfica con reconocimiento de la fuente. This document is part of the digital collection of the Central Library Dr. Luis Federico Leloir, available in bibliotecadigital.exactas.uba.ar. It should be used accompanied by the corresponding citation acknowledging the source. Cita tipo APA: Cruz, Julio César. (2017). Histomorfología de la glándula tiroides durante la ontogenia en Pseudis paradoxa (Anura, Hylidae). Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. https://hdl.handle.net/20.500.12110/tesis_n6259_Cruz Cita tipo Chicago: Cruz, Julio César. "Histomorfología de la glándula tiroides durante la ontogenia en Pseudis paradoxa (Anura, Hylidae)". Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. 2017. https://hdl.handle.net/20.500.12110/tesis_n6259_Cruz Dirección: Biblioteca Central Dr. Luis F. Leloir, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. Contacto: bibliotecadigital.exactas.uba.ar Intendente Güiraldes 2160 - C1428EGA - Tel. (++54 +11) 4789-9293 UNIVERSIDAD DE BUENOS AIRES Facultad de Ciencias Exactas y Naturales Departamento de Biodiversidad y Biología Experimental Histomorfología de la glándula tiroides durante la ontogenia -

Linking Environmental Drivers with Amphibian Species Diversity in Ponds from Subtropical Grasslands
Anais da Academia Brasileira de Ciências (2015) 87(3): 1751-1762 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201520140471 www.scielo.br/aabc Linking environmental drivers with amphibian species diversity in ponds from subtropical grasslands DARLENE S. GONÇALVES1, LUCAS B. CRIVELLARI2 and CARLOS EDUARDO CONTE3*,4 1Programa de Pós-Graduação em Zoologia, Universidade Federal do Paraná, Caixa Postal 19020, 81531-980 Curitiba, PR, Brasil 2Programa de Pós-Graduação em Biologia Animal, Universidade Estadual Paulista, Rua Cristovão Colombo, 2265, Jardim Nazareth, 15054-000 São José do Rio Preto, SP, Brasil 3Universidade Federal do Paraná. Departamento de Zoologia, Caixa Postal 19020, 81531-980 Curitiba, PR, Brasil 4Instituto Neotropical: Pesquisa e Conservação. Rua Purus, 33, 82520-750 Curitiba, PR, Brasil Manuscript received on September 17, 2014; accepted for publication on March 2, 2015 ABSTRACT Amphibian distribution patterns are known to be influenced by habitat diversity at breeding sites. Thus, breeding sites variability and how such variability influences anuran diversity is important. Here, we examine which characteristics at breeding sites are most influential on anuran diversity in grasslands associated with Araucaria forest, southern Brazil, especially in places at risk due to anthropic activities. We evaluate the associations between habitat heterogeneity and anuran species diversity in nine body of water from September 2008 to March 2010, in 12 field campaigns in which 16 species of anurans were found. Of the seven habitat descriptors we examined, water depth, pond surface area and distance to the nearest forest fragment explained 81% of total species diversity. -

Notes on the Paradox Frog, Pseudis Paradoxa, in Bolivia
British Herpetological Society Bullton. No. 68. 1999 NOTES ON THE PARADOX FROG, PSEUDIS PARADOXA, IN BOLIVIA IGNACIO DE LA RIVA Department of Biodiversity and Evolutionary Biology Museo Nacional de Ciencias Naturales a Jose Gutierrez Abascal 2, 28006 Madrid, Spain INTRODUCTION The South American Paradox Frog, Pseudis paradoxa (Plate. 1) is primarily a dweller of open, lowland areas, where it inhabits marshes, ponds and other types of lentic water bodies. It has a discontinuous distribution from Colombia to Argentina. Inter- populational differences primarily in colour pattern, as well a in some other features, have led to the recognition of seven subspecies [P. p. bolbodactyla and P. p. fusca were recently proposed to be elevated to specific status (Caramaschi and Cruz, 1998)]. DISTRIBUTION AND SUBSPECIES IN BOLIVIA For taxonomic and biogeographical reasons, the lowlands of Bolivia are an interesting area with respect to this frog. The distribution of the species in this country is poorly known. The first Bolivian record of P. paradoxa was provided by Muller and Hellmich (1936) at San Fermiin, Department of Santa Cruz. Since then, it was reported at some other localities, mostly in the Department of Santa Cruz [see De la Riva (1990) and below]. It was interesting that it was also discovered at two localities in southeastern Peru (Duellman and Salas, 1991; Henle, 1992). These discoveries made plausible that it ranges throughout the intermediate area of extensive, suitable habitat of humid savannas in the Bolivian Department of Beni. However, there is a surprising scarcity of published records for this huge and relatively (by Bolivian standards) well surveyed zone. -

Diet Composition of Lysapsus Bolivianus Gallardo, 1961 (Anura, Hylidae) of the Curiaú Environmental Protection Area in the Amazonas River Estuary
Herpetology Notes, volume 13: 113-123 (2020) (published online on 11 February 2020) Diet composition of Lysapsus bolivianus Gallardo, 1961 (Anura, Hylidae) of the Curiaú Environmental Protection Area in the Amazonas river estuary Mayara F. M. Furtado1 and Carlos E. Costa-Campos1,* Abstract. Information on a species’ diet is important to determine habitat conditions and resources and to assess the effect of preys on the species distribution. The present study aimed describing the diet of Lysapsus bolivianus in the floodplain of the Curiaú Environmental Protection Area. Individuals of L. bolivianus were collected by visual search in the floodplain of the Curiaú River. A total of 60 specimens of L. bolivianus were euthanized with 2% lidocaine, weighed, and their stomachs were removed for diet analysis. A total of 3.020 prey items were recorded in the diet. The most representative preys were: Diptera (36.21%), Collembola (16.61%), and Hemiptera (8.31%), representing 73.20% of the total consumed prey. Based on the Index of Relative Importance for males, females, and juveniles, the most important items in the diet were Diptera and Collembola. The richness of preys recorded in the diet of L. bolivianus in the dry season was lower than that of the rainy season. Regarding prey abundance and richness, L. bolivianus can be considered a generalist species and a passive forager, with a diet dependent on the availability of preys in the environment. Keywords. Amphibia; Eastern Amazon, Niche overlap, Predation, Prey diversity, Pseudinae Introduction (Toft 1980; 1981; Donnelly, 1991; López et al., 2009; López et al., 2015). The diet of most anuran species is composed mainly of The genus Lysapsus Cope, 1862 is restricted to South arthropods and because of the opportunistic behaviour of America and comprises aquatic and semi-aquatic anurans many species, anurans are usually regarded as generalist inhabiting temporary or permanent ponds with large predators (Duellman and Trueb, 1994). -

Universidade Estadual De Campinas Instituto De Biologia
UNIVERSIDADE ESTADUAL DE CAMPINAS INSTITUTO DE BIOLOGIA KALEB PRETTO GATTO “COMPARATIVE MOLECULAR CYTOGENETICS OF GENUS Pseudis (ANURA, HYLIDAE) AND GENOMIC ANALYSIS OF THE SEX CHROMOSOMES OF Pseudis tocantins” “CITOGENÉTICA MOLECULAR COMPARATIVA DO GÊNERO Pseudis (ANURA, HYLIDAE) E ANÁLISE GENÔMICA DOS CROMOSSOMOS SEXUAIS DE Pseudis tocantins” Campinas 2018 KALEB PRETTO GATTO “COMPARATIVE MOLECULAR CYTOGENETICS OF GENUS Pseudis (ANURA, HYLIDAE) AND GENOMIC ANALYSIS OF THE SEX CHROMOSOMES OF Psesudis tocantins” “CITOGENÉTICA MOLECULAR COMPARATIVA DO GÊNERO Pseudis (ANURA, HYLIDAE) E ANÁLISE GENÔMICA DOS CROMOSSOMOS SEXUAIS DE Pseudis tocantins” Thesis presented to the Institute of Biology of the University of Campinas in partial fulfillment of the requirements for the degree of Doctor in Cell and Structural Biology, in the area of Cell Biology. Tese de doutorado apresentada ao Instituto de Biologia da Universidade Estadual de Campinas para a obtenção do título de Doutor em Biologia Celular e Estrutural, na área de Biologia Celular. Este arquivo digital corresponde à versão final da tese defendida pelo aluno Kaleb Pretto Gatto e orientada pela professora Dra. Luciana Bolsoni Lourenço Morandini. Orientadora: Profa. Dra. Luciana Bolsoni Lourenço Morandini Campinas 2018 3 4 BANCA EXAMINADORA Profa. Dra. Luciana Bolsoni Lourenço (Orientadora) Prof. Dr. Fausto Foresti Profa. Dra. Karen Ventura Profa. Dra. Cinthia Aguirre Brasileiro Profa. Dra. Mariana Lucio Lyra Os membros da Comissão Organizadora acima assinaram a Ata de Defesa, que se encontra no processo de vida acadêmica do aluno. 5 Dedico esse trabalho a todos os mestres que, de alguma forma, me fizeram ter a paixão pela ciência e a vontade de desvendar o desconhecido na natureza. 6 Agradecimentos Agradeço a minha orientadora, professora Dra. -

Ecological Functions of Neotropical Amphibians and Reptiles: a Review
Univ. Sci. 2015, Vol. 20 (2): 229-245 doi: 10.11144/Javeriana.SC20-2.efna Freely available on line REVIEW ARTICLE Ecological functions of neotropical amphibians and reptiles: a review Cortés-Gomez AM1, Ruiz-Agudelo CA2 , Valencia-Aguilar A3, Ladle RJ4 Abstract Amphibians and reptiles (herps) are the most abundant and diverse vertebrate taxa in tropical ecosystems. Nevertheless, little is known about their role in maintaining and regulating ecosystem functions and, by extension, their potential value for supporting ecosystem services. Here, we review research on the ecological functions of Neotropical herps, in different sources (the bibliographic databases, book chapters, etc.). A total of 167 Neotropical herpetology studies published over the last four decades (1970 to 2014) were reviewed, providing information on more than 100 species that contribute to at least five categories of ecological functions: i) nutrient cycling; ii) bioturbation; iii) pollination; iv) seed dispersal, and; v) energy flow through ecosystems. We emphasize the need to expand the knowledge about ecological functions in Neotropical ecosystems and the mechanisms behind these, through the study of functional traits and analysis of ecological processes. Many of these functions provide key ecosystem services, such as biological pest control, seed dispersal and water quality. By knowing and understanding the functions that perform the herps in ecosystems, management plans for cultural landscapes, restoration or recovery projects of landscapes that involve aquatic and terrestrial systems, development of comprehensive plans and detailed conservation of species and ecosystems may be structured in a more appropriate way. Besides information gaps identified in this review, this contribution explores these issues in terms of better understanding of key questions in the study of ecosystem services and biodiversity and, also, of how these services are generated. -

Pseudis Paradoxa
__all_short_notes_sHoRt_note.qxd 12.02.2016 10:37 seite 26 192 sHoRt note HeRPetoZoa 28 (3/4) Wien, 30. Jänner 2016 sHoRt note Pseudis paradoxa (Linnaeus , 1758 ): um body size (svL 45-75 mm), relatively northward extension of the known small head and protruding eyes in dorsolat - distribution range in Colombia eral position with a yellow iris crossed by a brown bar ( LesCuRe & M aRty 2000). its common name makes reference to the extra - in spite of increasing knowledge about ordinary size of the tadpole relative to that the diversity of anuran amphibians in Co - of adult individuals ( eMeRson 1988). the lombia ( BeRnaL & L ynCH 2008; GaLvis - distribution of this species covers Colom - PeñueLa et al. 2011; aCosta -G aLvis 2012), bia, Guyana, suriname, Brazil, Bolivia, there are still many gaps with regard to the Peru, venezuela and the islands of trinidad known distribution of the species. Detec- and tobago ( FRost 2014). in Colombia, it tion of the presence of anuran species in is present in the the lower Rio Magdalena places from which they were previously area (Departments of antioquia, atlántico, unrecorded establishes a baseline for the Bolivar, Cesar, Cordoba, Magdalena, study of species richness, optimizing con - santander, sucre) as well as the departments servation plans, and research in the fields of of arauca and Meta in the eastern region of taxonomy, systematics and natural history. Colombia (Fig. 1a) ( RenGiFo & L unDBeRG this is the first report on the occurrence of 1999; aCosta -G aLvis 2000; Cuentas - the hylid frog Pseudis paradoxa (Linnaeus , MontaLvo 2002; aCosta -G aLvis 2014). 1758 ) in the north Colombian department of the authors recorded several individu - Guajira (Fig. -

Exceptional Fossil Preservation During CO2 Greenhouse Crises? Gregory J
Palaeogeography, Palaeoclimatology, Palaeoecology 307 (2011) 59–74 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Exceptional fossil preservation during CO2 greenhouse crises? Gregory J. Retallack Department of Geological Sciences, University of Oregon, Eugene, Oregon 97403, USA article info abstract Article history: Exceptional fossil preservation may require not only exceptional places, but exceptional times, as demonstrated Received 27 October 2010 here by two distinct types of analysis. First, irregular stratigraphic spacing of horizons yielding articulated Triassic Received in revised form 19 April 2011 fishes and Cambrian trilobites is highly correlated in sequences in different parts of the world, as if there were Accepted 21 April 2011 short temporal intervals of exceptional preservation globally. Second, compilations of ages of well-dated fossil Available online 30 April 2011 localities show spikes of abundance which coincide with stage boundaries, mass extinctions, oceanic anoxic events, carbon isotope anomalies, spikes of high atmospheric carbon dioxide, and transient warm-wet Keywords: Lagerstatten paleoclimates. Exceptional fossil preservation may have been promoted during unusual times, comparable with fi Fossil preservation the present: CO2 greenhouse crises of expanding marine dead zones, oceanic acidi cation, coral bleaching, Trilobite wetland eutrophication, sea level rise, ice-cap melting, and biotic invasions. Fish © 2011 Elsevier B.V. All rights reserved. Carbon dioxide Greenhouse 1. Introduction Zeigler, 1992), sperm (Nishida et al., 2003), nuclei (Gould, 1971)and starch granules (Baxter, 1964). Taphonomic studies of such fossils have Commercial fossil collectors continue to produce beautifully pre- emphasized special places where fossils are exceptionally preserved pared, fully articulated, complex fossils of scientific(Simmons et al., (Martin, 1999; Bottjer et al., 2002). -
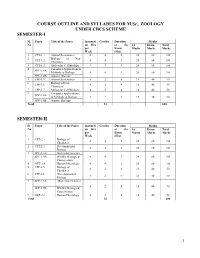
COURSE OUTLINE and SYLLABUS for M.Sc., ZOOLOGY UNDER CBCS SCHEME SEMESTER-I
COURSE OUTLINE AND SYLLABUS FOR M.Sc., ZOOLOGY UNDER CBCS SCHEME SEMESTER-I Sl. Paper Title of the Paper Instructi Credits Duration Marks No on Hrs of the IA Exam Total per Exam Marks Marks Marks Week (Hrs) 1 CPT-1.1 Animal Systematics 4 4 3 20 80 100 2 Biology of Non- CPT-1.2 4 4 3 20 80 100 Chordates 3 CPT-1.3 Molecular Cell Biology 4 4 3 20 80 100 4 Computer Applications & SPT-1.4A Methods in Biology 4 4 3 20 80 100 SPT-1.4B Aquatic Biology 5 CPP-1.5 Animal Systematics 4 2 4 10 40 50 6 Biology of Non- CPP-1.6 4 2 4 10 40 50 Chordates 7 CPP-1.7 Molecular Cell Biology 4 2 4 10 40 50 8 Computer Applications SPP-1.8A & Methods in Biology 4 2 4 10 40 50 SPP-1.8B Aquatic Biology Total 24 600 SEMESTER-II Sl. Paper Title of the Paper Instructi Credits Duration Marks No on Hrs of the IA Exam Total per Exam Marks Marks Marks Week (Hrs) 1 CPT-2.1 Biology of 4 4 3 20 80 100 Chordates 2 CPT-2.2 Developmental 4 4 3 20 80 100 Biology 3 SPT-2.3A Molecular Genetics SPT-2.3B Wildlife Biology & 4 4 3 20 80 100 Conservation 4 OET-2.4 Human Physiology 4 4 3 20 80 100 5 CPP-2.5 Biology of 4 2 4 10 40 50 Chordates 6 CPP-2.6 Developmental 4 2 4 10 40 50 Biology 7 SPP-2.7A Molecular Genetics 4 2 4 10 40 50 SPP-2.7B Wildlife Biology & Conservation 8 OEP-2.8 Human Physiology 4 2 4 10 40 50 Total 24 600 1 SEMESTER-III Sl. -

ROCEK, Z. and WUTTKE, M. (2010) Amphibia of Enspel (Late
Palaeobio Palaeoenv (2010) 90:321–340 DOI 10.1007/s12549-010-0042-0 ORIGINAL PAPER Amphibia of Enspel (Late Oligocene, Germany) ZbyněkRoček & Michael Wuttke Received: 23 April 2010 /Revised: 9 July 2010 /Accepted: 12 August 2010 /Published online: 29 September 2010 # Senckenberg Gesellschaft für Naturforschung and Springer 2010 Abstract Amphibia from the Late Oligocene (MP 28) One specimen is a large premetamorphic tadpole (no locality Enspel, Germany are represented by two caudates: rudimentary limbs) with a total body length of 147 mm. a hyperossified salamandrid Chelotriton paradoxus and an Anatomically, it can be equally assigned to Pelobates or to indeterminate salamandrid different from Chelotriton in Eopelobates; the second possibility was excluded only on proportions of vertebral column. Anurans are represented the basis of absence of adult Eopelobates in this locality. by two forms of the genus Palaeobatrachus, one of which is nearly as large as P. gigas (now synonymized with P. Keywords Enspel . Oligocene . Salamandridae . grandipes). Pelobates cf. decheni, represented in this Chelotriton . Anura . Palaeobatrachus . Pelobates . Rana locality by three nearly complete adult skeletons and a large number of tadpoles, is the earliest record for the Abbreviation genus. Compared with later representatives of the genus, it DP FNSP Department of Palaeontology Faculty of does not yet possess specializations for burrowing. Ranidae Natural Sciences, Prague are represented by two rather fragmentary and incomplete skeletons referred to as Rana sp. A comparatively large series of tadpoles was assigned to the Pelobatidae on the basis of tripartite frontoparietal complex. Most of them are Introduction premetamorphic larvae, and a few older ones are post- metamorphic, but they do not exceed Gossner stage 42.