Larval Development in Oligocene Palaeobatrachid Frogs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Para Conhecer a Terra: Memórias E Notícias De Geociências No Espaço Lusófono Autor(Es): Lopes, F.C. (Coord.); Andrade, A. I
Para conhecer a Terra: memórias e notícias de Geociências no espaço lusófono Lopes, F.C. (coord.); Andrade, A. I. (coord.); Henriques, M. H. (coord.); Autor(es): Quinta-Ferreira, M. (coord.); Reis, R. Pena dos (coord.); Barata, M. T. (coord.) Publicado por: Imprensa da Universidade de Coimbra URL persistente: URI:http://hdl.handle.net/10316.2/24406 DOI: DOI:http://dx.doi.org/10.14195/978-989-26-0534-0 Accessed : 11-Oct-2021 03:52:55 A navegação consulta e descarregamento dos títulos inseridos nas Bibliotecas Digitais UC Digitalis, UC Pombalina e UC Impactum, pressupõem a aceitação plena e sem reservas dos Termos e Condições de Uso destas Bibliotecas Digitais, disponíveis em https://digitalis.uc.pt/pt-pt/termos. Conforme exposto nos referidos Termos e Condições de Uso, o descarregamento de títulos de acesso restrito requer uma licença válida de autorização devendo o utilizador aceder ao(s) documento(s) a partir de um endereço de IP da instituição detentora da supramencionada licença. Ao utilizador é apenas permitido o descarregamento para uso pessoal, pelo que o emprego do(s) título(s) descarregado(s) para outro fim, designadamente comercial, carece de autorização do respetivo autor ou editor da obra. Na medida em que todas as obras da UC Digitalis se encontram protegidas pelo Código do Direito de Autor e Direitos Conexos e demais legislação aplicável, toda a cópia, parcial ou total, deste documento, nos casos em que é legalmente admitida, deverá conter ou fazer-se acompanhar por este aviso. pombalina.uc.pt digitalis.uc.pt 9 789892 605111 Série Documentos A presente obra reúne um conjunto de contribuições apresentadas no I Congresso Imprensa da Universidade de Coimbra Internacional de Geociências na CPLP, que decorreu de 14 a 16 de maio de 2012 no Coimbra University Press Auditório da Reitoria da Universidade de Coimbra. -

Biostratigraphy and Paleoecology of Continental Tertiary Vertebrate Faunas in the Lower Rhine Embayment (NW-Germany)
Netherlands Journal of Geosciences / Geologie en Mijnbouw 81 (2): 177-183 (2002) Biostratigraphy and paleoecology of continental Tertiary vertebrate faunas in the Lower Rhine Embayment (NW-Germany) Th. Mors Naturhistoriska Riksmuseet/Swedish Museum of Natural History, Department of Palaeozoology, P.O. Box 50007, SE-104 05 Stockholm, Sweden; e-mail: [email protected] Manuscript received: October 2000; accepted: January 2002 ^ Abstract This paper discusses the faunal content, the mammal biostratigraphy, and the environmental ecology of three important con tinental Tertiary vertebrate faunas from the Lower Rhine Embayment. The sites investigated are Rott (MP 30, Late Oligocene), Hambach 6C (MN 5, Middle Miocene), Frechen and Hambach 11 (both MN 16, Late Pliocene). Comparative analysis of the entire faunas shows the assemblages to exhibit many conformities in their general composition, presumably re sulting from their preference for wet lowlands. It appears that very similar environmental conditions for vertebrates reoc- curred during at least 20 Ma although the sites are located in a tectonically active region with high subsidence rates. Differ ences in the faunal composition are partly due to local differences in the depositional environment of the sites: lake deposits at the margin of the embayment (Rott), coal swamp and estuarine conditions in the centre of the embayment (Hambach 6C), and flood plain environments with small rivulets (Frechen and Hambach 1 l).The composition of the faunal assemblages (di versity and taxonomy) also documents faunal turnovers with extinctions and immigrations (Oligocene/Miocene and post- Middle Miocene), as a result of changing climate conditions. Additional vertebrate faunal data were retrieved from two new assemblages collected from younger strata at the Hambach mine (Hambach 11C and 14). -

Baez NHM 1997.Pdf (3.622Mb)
HERP QL 668 B33 l':iif;,O0i.U'.Z06l("'^ ocxentxpc Papers Natural History Museum The University of Kansas 29 October 1997 Number 4:1^1 Redescription of the Paleogene Shelania pascuali from Patagonia and Its Bearing on the Relationships of Fossil and Recent Pipoid Frogs >. By o O Ana Maria BAez^ and Linda Trueb- o ^Departamento de Geologia, FacuUad de Ciencias Exactas, Universidad de 0) w >. > t- Buenos Aires, Pabellon II, Ciudad Universitaria, 1428 Buenos Aires, Argentina CO !-> >^ ^ £ -^ '^Division of Herpetology, Natural Histoiy Museum, and Department of P Kansas 66045-2454, J3 o, Systematics and Ecology, The University of Kansas, Lawrence, USA a o t3 o CO ^ t. CONTENTS ° CO I ABSTRACT 2 « RESUMEN 2 5S INTRODUCTION 2 Previous Paleontological Work 4 Acknowledgments 4 MATERIALS AND METHODS 5 General Methodology 5 Cladistic Methodology 5 Specimens Examined 6 STRATIGRAPHIC PROVENANCE AND AGE OF MATERIAL 6 REDESCRIPTION OF SHELANIA 8 ANALYSIS OF CHARACTERS 16 RESULTS 31 DISCUSSION 35 Taxonomic Considerations 35 Characters 36 LITERATURE CITED 37 APPENDIX 40 © Natural History Museum, The University of Kansas ISSN No. 1094-0782 — 2 Scientific Papers, Natural History Museum, The University of Kansas 1960, is redescribed on the basis of a series of 30 recently I ABSTIMCT Shdania pascuali Casamiquela, .'-\ ' . discovered specimens, which range in estimated snout-vent length from 30-100 mm, from the Paleo- y ' gene of Patagonia. This large pipoid anuran is distinguished by possessing a long, narrow braincase; an hourglass-shaped frontoparietal; a robust antorbital process on the edentate maxilla; long, straight {/) '/^ I ilia that describe a V-shape in dorsal profile; and a trunk that is long relative to the lengths of the head and limbs. -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

Changes of the Palynobiotas in the Mesozoic and Cenozoic of Patagonia: a Review
Biological Journal of the Linnean Society, 2011, 103, 380–396. With 6 figures Changes of the palynobiotas in the Mesozoic and Cenozoic of Patagonia: a review MIRTA E. QUATTROCCHIO1,2*, WOLFGANG VOLKHEIMER3, ANA MARÍA BORRROMEI1 and MARCELO A. MARTÍNEZ1,2 1INGEOSUR, CONICET, San Juan 670, (8000), Bahía Blanca, Argentina 2Departamento de Geología, Universidad Nacional del Sur, San Juan 670, (8000) Bahía Blanca, Argentina 3IANIGLA, CCT-CONICET, CC330 (5500) Mendoza, Argentina Received 22 February 2011; accepted for publication 22 February 2011bij_1652 380..396 This study describes the compositional changes of Mesozoic–Cenozoic palynologic assemblages in Patagonia, the succession of phytogeographic scenarios and some evolutionary key events. The Triassic ‘Ipswich Microflora’, characterized by bisaccate pollen and high frequencies of trilete spores, represents the warm–temperate province of south-western Gondwana (Dicroidium Flora), followed in time, in Patagonia, near the Triassic/Jurassic bound- ary, by the ‘Classopollis Microflora’, indicating warm and (seasonal) arid conditions. Araucariaceans and podocar- paceans grew on Jurassic uplands. The exclusively Early Cretaceous taxa Cyclusphaera and Balmeiopsis (Coniferae) appeared in the Valanginian. The oldest angiospermous pollen types of Argentina appear in the Barremian–Aptian of southern Patagonia. During the Maastrichtian, the Nothofagaceae and Myrtaceae are incoming. In the Palaeocene, a vegetation dominated by tropical elements would have developed in Patagonia. Increasing Nothofagidites frequency in northern Patagonia during the Middle to Late Eocene indicates climatic change from warm to temperate. Of three Early to Middle Miocene phytogeographic provinces (Neotropical– Transitional and Austral), the Transitional one was replaced during the Late Miocene–Pliocene by the xerophytic Proto Espinal/Steppe with a shrubby–herbaceous vegetation. -

A Review of Neusibatrachus Wilferti, an Early Cretaceous Frog from the Montsec Range, Northeastern Spain
A review of Neusibatrachus wilferti, an Early Cretaceous frog from the Montsec Range, northeastern Spain ANA M. BÁEZ and BORJA SANCHIZ Báez, A.M. and Sanchiz, B. 2007. A review of Neusibatrachus wilferti, an Early Cretaceous frog from the Montsec Range, northeastern Spain. Acta Palaeontologica Polonica 52 (3): 477–487. Neusibatrachus wilferti is an anuran from the late Berriasian–early Valanginian fossiliferous lacustrine limestones that are exposed in the eastern part of the Montsec Range, province of Lleida, Spain. It was originally described by Seiffert in 1972 and its phylogenetic position has since been discussed. Neusibatrachus has been considered an undeterminable fos− sil, an abnormal individual, or a primitive palaeobatrachid. Here we redescribe the only available specimen, and clarify features, such as absence of palatines, nine presacrals, and procoelous vertebral centra, that have been the subject of previ− ous debates. We consider the specimen to be a postmetamorphic individual and make developmental interpretations of some of its characters. In particular, we provide evidence of a living anuran (Rana iberica) that resembles Neusibatrachus in the development of intervertebral articulations. Neusibatrachus is considered a valid genus, which differs from other anurans, except for the pipoids, in the joint presence of an azygous frontoparietal and a parasphenoid lacking the subotic alae, although it differs from the pipoids in having nine presacral vertebrae. Morphological evidence indicates that Neusi− batrachus is related to Xenoanura, the pipoid branch in the living Amphibia Tree of Life based on molecular data. More− over, it might be a member of the pipoid clade proper, which presently includes the Pipidae, Rhinophrynidae, and several fossil taxa, including the Palaeobatrachidae, although the evidence is not conclusive. -

Exceptional Fossil Preservation During CO2 Greenhouse Crises? Gregory J
Palaeogeography, Palaeoclimatology, Palaeoecology 307 (2011) 59–74 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Exceptional fossil preservation during CO2 greenhouse crises? Gregory J. Retallack Department of Geological Sciences, University of Oregon, Eugene, Oregon 97403, USA article info abstract Article history: Exceptional fossil preservation may require not only exceptional places, but exceptional times, as demonstrated Received 27 October 2010 here by two distinct types of analysis. First, irregular stratigraphic spacing of horizons yielding articulated Triassic Received in revised form 19 April 2011 fishes and Cambrian trilobites is highly correlated in sequences in different parts of the world, as if there were Accepted 21 April 2011 short temporal intervals of exceptional preservation globally. Second, compilations of ages of well-dated fossil Available online 30 April 2011 localities show spikes of abundance which coincide with stage boundaries, mass extinctions, oceanic anoxic events, carbon isotope anomalies, spikes of high atmospheric carbon dioxide, and transient warm-wet Keywords: Lagerstatten paleoclimates. Exceptional fossil preservation may have been promoted during unusual times, comparable with fi Fossil preservation the present: CO2 greenhouse crises of expanding marine dead zones, oceanic acidi cation, coral bleaching, Trilobite wetland eutrophication, sea level rise, ice-cap melting, and biotic invasions. Fish © 2011 Elsevier B.V. All rights reserved. Carbon dioxide Greenhouse 1. Introduction Zeigler, 1992), sperm (Nishida et al., 2003), nuclei (Gould, 1971)and starch granules (Baxter, 1964). Taphonomic studies of such fossils have Commercial fossil collectors continue to produce beautifully pre- emphasized special places where fossils are exceptionally preserved pared, fully articulated, complex fossils of scientific(Simmons et al., (Martin, 1999; Bottjer et al., 2002). -
COURSE OUTLINE and SYLLABUS for M.Sc., ZOOLOGY UNDER CBCS SCHEME SEMESTER-I
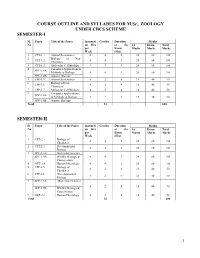
COURSE OUTLINE AND SYLLABUS FOR M.Sc., ZOOLOGY UNDER CBCS SCHEME SEMESTER-I Sl. Paper Title of the Paper Instructi Credits Duration Marks No on Hrs of the IA Exam Total per Exam Marks Marks Marks Week (Hrs) 1 CPT-1.1 Animal Systematics 4 4 3 20 80 100 2 Biology of Non- CPT-1.2 4 4 3 20 80 100 Chordates 3 CPT-1.3 Molecular Cell Biology 4 4 3 20 80 100 4 Computer Applications & SPT-1.4A Methods in Biology 4 4 3 20 80 100 SPT-1.4B Aquatic Biology 5 CPP-1.5 Animal Systematics 4 2 4 10 40 50 6 Biology of Non- CPP-1.6 4 2 4 10 40 50 Chordates 7 CPP-1.7 Molecular Cell Biology 4 2 4 10 40 50 8 Computer Applications SPP-1.8A & Methods in Biology 4 2 4 10 40 50 SPP-1.8B Aquatic Biology Total 24 600 SEMESTER-II Sl. Paper Title of the Paper Instructi Credits Duration Marks No on Hrs of the IA Exam Total per Exam Marks Marks Marks Week (Hrs) 1 CPT-2.1 Biology of 4 4 3 20 80 100 Chordates 2 CPT-2.2 Developmental 4 4 3 20 80 100 Biology 3 SPT-2.3A Molecular Genetics SPT-2.3B Wildlife Biology & 4 4 3 20 80 100 Conservation 4 OET-2.4 Human Physiology 4 4 3 20 80 100 5 CPP-2.5 Biology of 4 2 4 10 40 50 Chordates 6 CPP-2.6 Developmental 4 2 4 10 40 50 Biology 7 SPP-2.7A Molecular Genetics 4 2 4 10 40 50 SPP-2.7B Wildlife Biology & Conservation 8 OEP-2.8 Human Physiology 4 2 4 10 40 50 Total 24 600 1 SEMESTER-III Sl. -

ROCEK, Z. and WUTTKE, M. (2010) Amphibia of Enspel (Late
Palaeobio Palaeoenv (2010) 90:321–340 DOI 10.1007/s12549-010-0042-0 ORIGINAL PAPER Amphibia of Enspel (Late Oligocene, Germany) ZbyněkRoček & Michael Wuttke Received: 23 April 2010 /Revised: 9 July 2010 /Accepted: 12 August 2010 /Published online: 29 September 2010 # Senckenberg Gesellschaft für Naturforschung and Springer 2010 Abstract Amphibia from the Late Oligocene (MP 28) One specimen is a large premetamorphic tadpole (no locality Enspel, Germany are represented by two caudates: rudimentary limbs) with a total body length of 147 mm. a hyperossified salamandrid Chelotriton paradoxus and an Anatomically, it can be equally assigned to Pelobates or to indeterminate salamandrid different from Chelotriton in Eopelobates; the second possibility was excluded only on proportions of vertebral column. Anurans are represented the basis of absence of adult Eopelobates in this locality. by two forms of the genus Palaeobatrachus, one of which is nearly as large as P. gigas (now synonymized with P. Keywords Enspel . Oligocene . Salamandridae . grandipes). Pelobates cf. decheni, represented in this Chelotriton . Anura . Palaeobatrachus . Pelobates . Rana locality by three nearly complete adult skeletons and a large number of tadpoles, is the earliest record for the Abbreviation genus. Compared with later representatives of the genus, it DP FNSP Department of Palaeontology Faculty of does not yet possess specializations for burrowing. Ranidae Natural Sciences, Prague are represented by two rather fragmentary and incomplete skeletons referred to as Rana sp. A comparatively large series of tadpoles was assigned to the Pelobatidae on the basis of tripartite frontoparietal complex. Most of them are Introduction premetamorphic larvae, and a few older ones are post- metamorphic, but they do not exceed Gossner stage 42. -

Rampant Tooth Loss Across 200 Million Years of Frog Evolution
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.04.429809; this version posted February 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Rampant tooth loss across 200 million years of frog evolution 2 3 4 Daniel J. Paluh1,2, Karina Riddell1, Catherine M. Early1,3, Maggie M. Hantak1, Gregory F.M. 5 Jongsma1,2, Rachel M. Keeffe1,2, Fernanda Magalhães Silva1,4, Stuart V. Nielsen1, María Camila 6 Vallejo-Pareja1,2, Edward L. Stanley1, David C. Blackburn1 7 8 1Department of Natural History, Florida Museum of Natural History, University of Florida, 9 Gainesville, Florida USA 32611 10 2Department of Biology, University of Florida, Gainesville, Florida USA 32611 11 3Biology Department, Science Museum of Minnesota, Saint Paul, Minnesota USA 55102 12 4Programa de Pós Graduação em Zoologia, Universidade Federal do Pará/Museu Paraense 13 Emilio Goeldi, Belém, Pará Brazil 14 15 *Corresponding author: Daniel J. Paluh, [email protected], +1 814-602-3764 16 17 Key words: Anura; teeth; edentulism; toothlessness; trait lability; comparative methods 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.04.429809; this version posted February 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. -

Frontoparietal Bone in Extinct Palaeobatrachidae (Anura): Its Variation and Taxonomic Value
THE ANATOMICAL RECORD 298:1848–1863 (2015) Frontoparietal Bone in Extinct Palaeobatrachidae (Anura): Its Variation and Taxonomic Value ZBYNEK ROCEK, 1* RENAUD BOISTEL,2 NICOLAS LENOIR,3 ARNAUD MAZURIER,4 STEPHANIE E. PIERCE,5 JEAN-CLAUDE RAGE,6 SERGEI V. SMIRNOV,7 ACHIM H. SCHWERMANN,8 XAVIER VALENTIN,2 9 10 11 MARTON VENCZEL, MICHAEL WUTTKE, AND TOMA S ZIKMUND 1Department of Palaeobiology, Geological Institute, Academy of Sciences of the Czech Republic, Prague, Czech Republic 2Institut International de Paleoprimatologie et de Paleontologie Humaine, UMR 7262 CNRS, Universite de Poitiers, Poitiers, France 3Multiscale Group, Laboratoire Navier, UMR8205-CNRS/ENPC/IFSTTAR/Universite Paris-Est, Champs-sur-Marne, France 4Institut de Chimie des Milieux et Materiaux de Poitiers, UMR 7285 Universitede Poitiers, UFR SFA, Poitiers, France 5Department of Organismic and Evolutionary Biology and Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts 6Sorbonne Universites—CR2P—MNHN, CNRS, UPMC-Paris 6, Museum National d’Histoire Naturelle, Paris, France 7Laboratory of Evolutionary Morphology, a.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow, Russia 8Steinmann-Institut fur€ Geologie, Mineralogie und Palaontologie,€ Universitat€ Bonn, Bonn, Germany 9 T; arii Cris¸urilor Museum, Oradea, Romania 10Department of Archaeology, General Department of Cultural Heritage Rhineland Palatinate, Section Geological History of the Earth, Mainz, Germany 11X-Ray Micro CT and Nano CT Research Group, CEITEC—Central European Institute of Technology, Brno University of Technology, Brno, Czech Republic ABSTRACT Palaeobatrachidae are extinct frogs from Europe closely related to the Gondwanan Pipidae, which includes Xenopus. Their frontoparietal is a distinctive skeletal element which has served as a basis for establishing the genus Albionbatrachus. -

From the Early Cretaceous Crato Formation
Journal of South American Earth Sciences 92 (2019) 222–233 Contents lists available at ScienceDirect Journal of South American Earth Sciences journal homepage: www.elsevier.com/locate/jsames A new genus of pipimorph frog (Anura) from the Early Cretaceous Crato T Formation (Aptian) and the evolution of South American tongueless frogs ∗ ∗∗ Ismar Souza Carvalhoa, , Federico Agnolinb,c, , Mauro A. Aranciaga Rolandob, Fernando E. Novasb, José Xavier-Netod, Francisco Idalécio Freitase, José Artur Ferreira Gomes Andradef a Universidade Federal do Rio de Janeiro, Departamento de Geologia, CCMN/IGEO 21.949-900 Cidade Universitária - Ilha do Fundão, Rio de Janeiro, Brazil b Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Consejo Nacional de Investigaciones Científicas y Técnicas – CONICET, Buenos Aires, Argentina c Fundación de Historia Natural ‘Félix de Azara’, Universidad Maimónides, Buenos Aires, Argentina d Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasília (DF), Brazil e Geopark Araripe, Rua Carolino Sucupira s/n, Pimenta, 105 Centro, 63.100-490 Ceará, Brazil f Departamento Nacional da Produção Mineral, Ceará, Praça da Sé, 105 Centro, 63.100-440 Ceará, Brazil ARTICLE INFO ABSTRACT Keywords: Pipimorpha is a clade of tongueless anurans with a wide fossil record. Furthermore, the oldest South American Crato Formation fossils come from the Late Cretaceous (Cenomanian) of Patagonia, Argentina. The aim of the present con- Lower Cretaceous tribution is to describe a new genus and species of Pipimorpha from the Crato Formation (Aptian, Early Pipimorpha Cretaceous), Araripe Basin, Brazil. The new specimen consists of a nearly complete skeleton that shows several Brazil anatomical similarities with other fossils from South America.