Giant Intraosseous Cyst-Like Lesions of the Metacarpal Bones in Rheumatoid Arthritis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Four Unusual Cases of Congenital Forelimb Malformations in Dogs
animals Article Four Unusual Cases of Congenital Forelimb Malformations in Dogs Simona Di Pietro 1 , Giuseppe Santi Rapisarda 2, Luca Cicero 3,* , Vito Angileri 4, Simona Morabito 5, Giovanni Cassata 3 and Francesco Macrì 1 1 Department of Veterinary Sciences, University of Messina, Viale Palatucci, 98168 Messina, Italy; [email protected] (S.D.P.); [email protected] (F.M.) 2 Department of Veterinary Prevention, Provincial Health Authority of Catania, 95030 Gravina di Catania, Italy; [email protected] 3 Institute Zooprofilattico Sperimentale of Sicily, Via G. Marinuzzi, 3, 90129 Palermo, Italy; [email protected] 4 Veterinary Practitioner, 91025 Marsala, Italy; [email protected] 5 Ospedale Veterinario I Portoni Rossi, Via Roma, 57/a, 40069 Zola Predosa (BO), Italy; [email protected] * Correspondence: [email protected] Simple Summary: Congenital limb defects are sporadically encountered in dogs during normal clinical practice. Literature concerning their diagnosis and management in canine species is poor. Sometimes, the diagnosis and description of congenital limb abnormalities are complicated by the concurrent presence of different malformations in the same limb and the lack of widely accepted classification schemes. In order to improve the knowledge about congenital limb anomalies in dogs, this report describes the clinical and radiographic findings in four dogs affected by unusual congenital forelimb defects, underlying also the importance of reviewing current terminology. Citation: Di Pietro, S.; Rapisarda, G.S.; Cicero, L.; Angileri, V.; Morabito, Abstract: Four dogs were presented with thoracic limb deformity. After clinical and radiographic S.; Cassata, G.; Macrì, F. Four Unusual examinations, a diagnosis of congenital malformations was performed for each of them. -
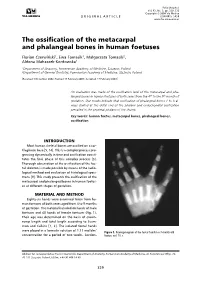
The Ossification of the Metacarpal and Phalangeal Bones in Human Foetuses
Folia Morphol. Vol. 63, No. 3, pp. 329–332 Copyright © 2004 Via Medica O R I G I N A L A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl The ossification of the metacarpal and phalangeal bones in human foetuses Florian Czerwiński1, Ewa Tomasik1, Małgorzata Tomasik2, Aldona Mahaczek-Kordowska1 1Department of Anatomy, Pomeranian Academy of Medicine, Szczecin, Poland 2Department of General Dentistry, Pomeranian Academy of Medicine, Szczecin, Poland [Received 4 November 2002; Revised 17 February 2004; Accepted 17 February 2004] An evaluation was made of the ossification level of the metacarpal and pha- langeal bones in human foetuses of both sexes from the 4th to the 9th month of gestation. Our results indicate that ossification of phalangeal bones 1 to 5 al- ways started at the distal end of the phalanx and endochondral ossification prevailed in the proximal phalanx of the thumb. Key words: human foetus, metacarpal bones, phalangeal bones, ossification INTRODUCTION Most human skeletal bones are ossified on a car- tilaginous base [5, 14]. This is a complex process pro- gressing dynamically in time and ossification consti- tutes the final phase of this complex process [3]. Thorough observation of the ossification of the foe- tal skeleton is made possible by means of the radio- logical method and evaluation of histological speci- mens [9]. This study presents the ossification of the metacarpal and phalangeal bones in human foetus- es at different stages of gestation. MATERIAL AND METHOD Eighty-six hands were examined taken from hu- man foetuses of both sexes aged from 4 to 9 months of gestation. -

Metacarpal Fractures: Practical Methods for Measurement of Shortening, Angulation, and Malrotation
J Orthop Spine Trauma. 2020 March; 6(1): 9-13. DOI: http://dx.doi.org/10.18502/jost.v6i1.4535 Educational Corner Metacarpal Fractures: Practical Methods for Measurement of Shortening, Angulation, and Malrotation Rohollah Khajeh 1, Behzad Enayati1, Farzad Vosughi2, Seyed Mohammad Javad Mortazavi 3,* 1 Fellowship of Hand Surgery, Joint Reconstruction Research Center, Tehran University of Medical Sciences, Tehran, Iran 2 Resident, Department of Orthopedics, Joint Reconstruction Research Center, Tehran University of Medical Sciences, Tehran, Iran 3 Professor, Department of Orthopedic Surgery, Joint Reconstruction Research Center, Tehran University of Medical Sciences, Tehran, Iran *Corresponding author: Seyed Mohammad Javad Mortazavi; Department of Orthopedic Surgery, Joint Reconstruction Research Center, Tehran University of Medical Sciences, Tehran, Iran. Tel: +98-2161192767, Email: [email protected] Received: 09 October 2019; Revised: 15 December 2019; Accepted: 17 January 2020 Keywords: Metacarpus; Fractures, Bone; Hand Deformities, Acquired Citation: Khajeh R, Enayati B, Vosughi F, Mortazavi SMJ. Metacarpal Fractures: Practical Methods for Measurement of Shortening, Angulation, and Malrotation. J Orthop Spine Trauma 2020; 6(1): 9-13. Background different metacarpal fractures. Articular fractures involving less than 20% of the joint Phalangeal, distal radius, and metacarpal fractures are surface and nondisplaced or minimally displaced shaft the most frequent upper limb fractures, respectively (1). fractures, without significant angulation, malrotation, or The incidence of metacarpal fractures in the United States shortening are treated with immobilization in the of America (USA) is 13.6 among every 100000 population intrinsic plus position (6). Bony apposition of at least 50% annually (2). Metacarpal fractures compose 30-40 percent and maximal bone shortening of 5 mm is acceptable. -

Macroanatomy of the Bones of Thoracic Limb of an Asian Elephant (Elephas Maximus)
Int. J. Morphol., 34(3):909-917, 2016. Macroanatomy of the Bones of Thoracic Limb of an Asian Elephant (Elephas maximus) Macroanatomía de los Huesos del Miembro Torácico de un Elefante Asiático (Elephas maximus) A. S. M. Lutful Ahasan*; Md. Abul Quasem*; Mohammad Lutfur Rahman*; Rubyath Binte Hasan*; A. S. M. Golam Kibria* & Subrata Kumar Shil* AHASAN, A. M. S. L.; QUASEM, M. A.; RAHMAN, M. L.; HASAN, R. B.; KIBRIA, A. S. M. G. & SHIL, S. K. Macroanatomy of the bones of thoracic limb of an Asian Elephant (Elephas maximus). Int. J. Morphol., 34(3):909-917, 2016. SUMMARY: Bones of forelimb were studied from a prepared skeleton of an adult female Asian elephant (Elephas maximus) in Anatomy Museum of Chittagong Veterinary and Animal Sciences University to understand the morphological form and structure of Asian elephant forelimb. The angle was approximately 123º between caudal border of scapula and caudal border of humerus. The scapula, humerus and bones of the antebrachium (particularly the ulna) were massive bones. The bones of manus were the short and relatively small. The dorsal border of scapula extended from the level of proximal extremity of first rib to the middle of the 6th rib. Ventral angle of scapula articulated with humerus by elongated shaped glenoid cavity (cavitas glenoidalis) of scapula and head of humerus (caput humeri). The major tubercle (tuberculum majus) of humerus was situated laterally to the head, which had smaller cranial part with large caudal part and extended cranially to the head. The crest of minor tubercle (tuberculum minus) was present as the rough line on the mediocaudal surface of humerus that ends in a slight depressed or elevated area, known as teres major tuberosity (tuberositas teres major). -
5Th Metacarpal Fractures Information for Patients
[Type here] Virtual fracture clinic 5th Metacarpal Fractures Information for patients What is a 5th metacarpal fracture? Your hand is made up of five metacarpal bones that connect your fingers and thumb to your wrist. A 5th metacarpal fracture (also known as a boxer’s fracture) is a break in the bone that connects your little finger to your wrist. A break is the same as a fracture. What causes a 5th metacarpal fracture? 5th metacarpal fractures usually occur when there is impact between a hand that is closed in a fist with a firm object, such as the ground or a wall. 5th metacarpal fractures are the most commonly occurring fractures in the hand. What are the signs and symptoms of a 5th metacarpal fracture? If you have fractured your 5th metacarpal bone you may notice that the back of your hand and the little finger are swollen and it will be more difficult to see the knuckles. You may also have some bruising and find it difficult to open and close your fingers. An x-ray should be taken to check the position of the fracture. What are the treatments available for a 5th metacarpal fracture? Most 5th metacarpal fractures are successfully treated without surgery. Even in cases where the alignment of the bone fragments is disrupted, the bones usually heal without any serious long term consequences. The options for non-surgical management are usually to: 1. ‘Buddy strap’ the little finger to the ring finger for two to four weeks and move it as pain allows 2. -

Wing Injuries
Wing Injuries Kimberly A McMunn MS MPH DVM – Anatomy Review – Radiological Positioning – Patient Evaluation – Approaches to Fracture Management – Physical Therapy – Monitoring Healing – Complications – Treatments for Specific Injuries Anatomy of the Wing Proctor and Lynch 1993 Wing Musculature- Ventral Proctor and Lynch 1993 Wing Musculature- Dorsal Proctor and Lynch 1993 Wing Musculature- Flight Proctor and Lynch 1993 Flight bones Scott 2016 Wing Vasculature Proctor and Lynch 1993 Bird bones vs mammal bones – Bone cortices thin and brittle but very strong, with high calcium content – Any defect in wall greatly reduces their strength – Less holding power (compared to mammals) for fixation hardware – Limited soft tissue over many long bones, very thin skin, bone fragments exteriorize easily Bird bones vs mammal bones – Pneumatic bones- humerus, and ulna in some species (Pelicans and CA condors) – Majority of callus tissue in healing is derived from the periosteal surface, and blood supply to the periosteum from surrounding soft tissues is very important. The IM circulation appears to be of less significance in avian bone healing than in mammals Radiographic Positioning 2 Orthogonal Views!! Scott 2016 Evaluation of the Patient – Evaluate signalment, history, and physical and orthopedic exams – Hands-off observation for mentation, posture, respiration and general appearance – Consider anesthesia or sedation for exam – Consider co-morbidities, including eye, head or intracoelomic trauma in wild birds, or malnutrition and associated poor bone -

The Skeletal and Muscular Systems
The Skeletal and Muscular Lesson 2 Systems Lesson 2 ASSIGNMENT 4: ORGANIZATION OF THE SKELETON Read in your textbook, Clinical Anatomy and Physiology for Veterinary Technicians, pages 153–160. Then read Assignment 4 in this study guide. In this section, we’ll focus on the anatomy of the skeletal system. We’ll examine the purpose of each bone, compare similar bones found in different animals, and correlate the structure of each bone with its function. The skeleton includes hundreds of bones. Fortunately for your study, many of these are duplicated within the body, and many are similar across species. Functions of Bone Stop and think about the bones in your body. What do they do? You might think they simply support the body and its organs. But your bones are much more active than that. They act as points of attachment for your muscles as well as levers for muscle action. Your skeleton is made up of your bones. Without this framework of bones, your muscles couldn’t move your body parts as efficiently or quickly. Imagine how difficult it would be to lift a heavy object without the help of your skeleton. No matter how strong your muscles may be, they don’t work well without your bones. Your bones strengthen your body against injury and protect your internal organs. For example, your brain is protected by your skull, and your heart and lungs are protected by your rib cage. Bones also have metabolic functions. Metabolic functions are processes that deal with the buildup or break- down of living cells for the purposes of providing energy and facilitating growth. -

Anatomical Characteristic of Forelimb Skeleton of Sumatran Rhino (Dicerorhinus Sumatrensis)
Proceeding of the 5th Congress of Asian Association of Veterinary Anatomists Bali- INDONESIA, February 12-13th, 2015 AH-03 Anatomical Characteristic of Forelimb Skeleton of Sumatran Rhino (Dicerorhinus sumatrensis) Nurhidayat*, Eni Puji Lestari, Chairun Nisa’, Danang Dwi Cahyadi, Supratikno Department of Anatomy Physiology and Pharmacology, Faculty of Veterinary Medicine, Bogor Agricultural University, Bogor 16680, Indonesia *Corresponding author: [email protected] Keywords: Sumatran rhino, anatomy, forelimb skeleton INTRODUCTION Sumatran rhino is one of the endangered animal species of Indonesia. This animal belongs to the order Perissodactyla, family Rhinocerotidae and genus Dicerorhinus. Although the animal’s weight reaches 1.000 kgs, Sumatran rhino is the smallest among family Rhinocerotidae (Van Strien 1974). To support the big size and rounded body, Sumatran rhino has relatively short legs with three toes on each. The body’s structure is adjusted with the animal’s behavior to move quickly, even to be able to climb the sheer cliffs. In general, the forelimb get bigger burden than that the hindlimb to perform the daily activities, support the weight of the body, neck and head, so that the field wider footprint pivot (De Blasé dan Martin 1981). Therefore, Sumatran rhino’s forelimb skeleton need to be studied to provide information about the relationship between the characteristics of the skeletal structure of the forelimb related to their function. MATERIALS AND METHODS The study was used a set of forelimb skeleton of female Sumatran rhino (named: Dusun) aged around 20 years old that received from Sumatran Rhino Sanctuary (SRS), Way Kambas National Park, Lampung, Indonesia. This study was done by observing the Sumatran rhino’s forelimb skeleton in detail. -

Morphometry and Density Analysis of the Fifth Metacarpal by Amrita Unnikumaran a Thesis Submitted in Partial Fulfillment Of
Morphometry and Density Analysis of the Fifth Metacarpal by Amrita Unnikumaran A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Biomedical Engineering Department of Medical Engineering College of Engineering University of South Florida Co-Major Professor: William E. Lee III, Ph.D. Co-Major Professor: Peter Simon, Ph.D. Robert Frisina, Ph.D. Sergio Gutierrez, Ph.D. Date of Approval: October 25, 2019 Keywords: Bone Geometry, Bone Density, Computerized Tomography Scan Study, Sexual Dimorphism Copyright © 2019, Amrita Unnikumaran Dedication I dedicate this work to my family for their immense support and encouragement. Acknowledgments I would like to express my sincere gratitude to my mentor Dr. Peter Simon and Dr. William Lee for their guidance and motivation which helped me succeed. I very grateful to Dr. Robert Frisina and Dr. Sergio Gutirezz for their valuable time and suggestions which kept me motivated. I would also like to extend my gratitude to University of South Florida and Foundation for Orthopaedic Research and Education for giving me the opportunity to learn and experience high quality research. Table of Contents List of Tables ................................................................................................................................... iii List of Figures ................................................................................................................................... v Abstract ......................................................................................................................................... -

Bones of the Upper Limbs Anatomy Team 434
Bones of The Upper Limbs Anatomy Team 434 Color Index: If you have any complaint or ▪ Important Points suggestion please don’t hesitate to contact us on: ▪ Helping notes [email protected] ▪ Explanation OBJECTIVES ● List the different bones of the UL. ● List the characteristic features of each bone. ● Differentiate between the bones of the right and left sides. ● List the articulations between the different bones. New Terms Term Meaning Example Processes A V-shaped indentation (act as the key of the joint) Coracoid process in the scapula Notch An indentation, (incision) on an edge or surface Radial notch in the ulna A hollow place (The Notch is not complete but the fossa is complete and both of them act as Radial fossa in the humerus Fossa the lock of the joint) Tubercles A nodule or a small rounded projection on a bone Dorsal tubercle in the radius A large prominence on a bone usually serving for the attachment of muscles or ligaments (is a Tibial tuberosity in the tibia Tuberosity bigger projection than the Tubercle) Groove A channel, a long narrow depression sure Intertubercular groove in the humerus Between bones (the place where the two parallel bones attach together by the interosseous Sharp medial interosseous Interosseous border membrane) in the radius The long and narrow upper edge, angle, or crest of something the lateral supracondylar ridge in the femur Ridge Spine Thick projecting ridge of bone Spine of the Scapula Articulation Meeting of two bones to make the joints Any type of joint usually serves as point of attachment for muscles, ligaments, and might form joints Radial styloid process (wrist joint) Styloid process union of scaphoid bone fracture إعادة إلتحام العظام ببعظها عشان ترجع للحالة الطبيعية Union of the bone Bones Process is a bigger projection is a bigger projection . -
Bones, Joints and Muscles of the Upper and Lower Limbs Study Guide
Comenius University in Bratislava Jessenius Faculty of Medicine in Martin Department of Anatomy BONES, JOINTS AND MUSCLES OF THE UPPER AND LOWER LIMBS STUDY GUIDE MUDr. Gabriela Hešková, PhD. Doc. MUDr. Desanka Výbohová, PhD. Doc. MUDr. Yvetta Mellová, CSc. Martin, 2018 2 Authors: MUDr. Gabriela Hešková, PhD. Doc. MUDr. Desanka Výbohová, PhD. Doc. MUDr. Yvetta Mellová, CSc. Authors themselves are responsible for the content and English of the chapters. Reviewers: Prof. MUDr. Marian Adamkov, CSc. MUDr. Mária Semáneková, PhD. Copyright © 2018 Authors of the Department of the Anatomy Jessenius Faculty of Medicine in Martin of the Comenius University in Bratislava All rights reserved. ISBN 978-80-8187-049-1 788081 870491 3 TABLE OF CONTENTS TABLE OF CONTENTS ...................................................................................................................................... 4 PREFACE .............................................................................................................................................................. 7 INTRODUCTION ................................................................................................................................................. 8 SHORT INTRODUCTION TO SKELETON OF THE UPPER LIMB AND LOWER LIMB ..................... 9 SKELETON OF THE UPPER LIMB ............................................................................................................... 10 SCAPULA ....................................................................................................................................................... -
Didelphis Albiventris)
Int. J. Morphol., 39(2):416-422, 2021. Anatomical Characteristics of the Bones of the Thoracic Limb of White-Eared Opossum (Didelphis albiventris) Características Anatómicas de los Huesos del Miembro Torácico de la Zarigüeya Oreja Blanca (Didelphis albiventris) Guilherme pereira Chiarello; Silvio Piresgomes; Tais Harumi De Castro Sasahara & Maria Angélica Miglino CHIARELLO, G. P.; PIRESGOMES, S.; HARUMI DE CASTRO SASAHARA, T. & MIGLINO, M. A. Anatomical characteristics of the bones of the thoracic limb of white-eared opossum (Didelphis albiventris). Int. J. Morphol., 39(2):416-422, 2021. SUMMARY: The skeleton of the thoracic limb is one of the key aspects for the understanding of the habits and movement of different mammalian species. Considering the gap about studies related to marsupial osteology, this work proposes to study the aspects inherent to the skeleton of opossums, with emphasis on the detailed anatomical description of the bones that form the thoracic limb. For this purpose, the bones of six specimens of possums of the species Didelphis albiventris were used. These small to medium sized marsupials inhabit a wide range of South America, living in several types of habitats, being commonly described as arboreal omnivores and have anthropic habits. For the execution of this study, the bone accidents perceptible in the specimens were identified by superficial palpation, which were then radiographed. The thoracic limb bones were prepared by boiling and drying in the sun. Finally, from the radiographic images and the prepared bones, a detailed description of the anatomy of the bone components of the thoracic limb of Didelphis albiventris was made, joining the previously obtained data of surface anatomy.