Rapid Interpretation of Chest X-Rays by Intensive Care Physicians And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
X-Ray Interpretation
Objectives Describe a systematic method for interpretation of chest and abdomen x-rays List findings to accurately identify common X-ray Interpretation pathology in chest & abdomen x-rays Describe a systematic method to approach the Denise Ramponi, DNP, FNP-C, ENP-BC, FAANP, FAEN important components in interpretation of upper & lower extremity x-rays Chest X-ray: Standard Views Lateral Film Postero-anterior (PA): (LAT) view can determine th On inspiration – diaphragm descends to 10 rib the anterior-posterior posteriorly structures along the axis of the body Normal LAT film Counting Ribs AP View - Portable http://www.lumen.luc.edu/lumen/MedEd/medicine/pulmonar/cxr/cxr_f.htm When the patient is unable to tolerate routine views with pts sitting or supine No participation from the patient Film is against the patient's back (supine) 1 Consolidation, Atelectasis, Chest radiograph Interstitial involvement Consolidation - any pathologic process that fills the alveoli with Left and right heart fluid, pus, blood, cells or other borders well defined substances Interstitial - involvement of the Both hemidiaphragms supporting tissue of the lung visible to midline parenchyma resulting in fine or coarse reticular opacities Right - higher Atelectasis - collapse of a part of Heart less than 50% of the lung due to a decrease in the amount of air resulting in volume diameter of the chest loss and increased density. Infiltrate, Consolidation vs. Congestive Heart Failure Atelectasis Fluid leaking into interstitium Kerley B 2 Kerley B lines Prominent interstitial markings Kerley lines Magnified CXR Cardiomyopathy & interstitial pulmonary edema Short 1-2 cm white lines at lung periphery horizontal to pleural surface Distended interlobular septa - secondary to interstitial edema. -

Update on Diagnosis and Treatment of Idiopathic Pulmonary Fibrosis
J Bras Pneumol. 2015;41(5):454-466 http://dx.doi.org/10.1590/S1806-37132015000000152 REVIEW ARTICLE Update on diagnosis and treatment of idiopathic pulmonary fibrosis José Baddini-Martinez1, Bruno Guedes Baldi2, Cláudia Henrique da Costa3, Sérgio Jezler4, Mariana Silva Lima5, Rogério Rufino3,6 1. Divisão de Pneumologia, Departamento de Clínica Médica, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brasil. 2. Divisão de Pneumologia, Instituto do Coração, Hospital das Clínicas, ABSTRACT Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brasil. Idiopathic pulmonary fibrosis is a type of chronic fibrosing interstitial pneumonia, of 3. Disciplina de Pneumologia e Tisiologia, unknown etiology, which is associated with a progressive decrease in pulmonary Faculdade de Ciências Médicas, function and with high mortality rates. Interest in and knowledge of this disorder have Universidade do Estado do Rio de grown substantially in recent years. In this review article, we broadly discuss distinct Janeiro, Rio de Janeiro, Brasil. aspects related to the diagnosis and treatment of idiopathic pulmonary fibrosis. We 4. Ambulatório de Pneumologia, Hospital list the current diagnostic criteria and describe the therapeutic approaches currently Ana Nery, Salvador, Brasil. available, symptomatic treatments, the action of new drugs that are effective in slowing 5. Ambulatório de Doenças Pulmonares Intersticiais, Hospital do Servidor the decline in pulmonary function, and indications for lung transplantation. Público Estadual de São Paulo, São Keywords: Idiopathic pulmonary fibrosis/diagnosis; Idiopathic pulmonary fibrosis/therapy; Paulo, Brasil. Idiopathic pulmonary fibrosis/rehabilitation. 6. Programa de Pós-Graduação em Ciências Médicas, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brasil. -

Diagnosing Pulmonary Embolism M Riedel
309 Postgrad Med J: first published as 10.1136/pgmj.2003.007955 on 10 June 2004. Downloaded from REVIEW Diagnosing pulmonary embolism M Riedel ............................................................................................................................... Postgrad Med J 2004;80:309–319. doi: 10.1136/pgmj.2003.007955 Objective testing for pulmonary embolism is necessary, embolism have a low long term risk of subse- quent VTE.2 5–7 because clinical assessment alone is unreliable and the consequences of misdiagnosis are serious. No single test RISK FACTORS AND RISK has ideal properties (100% sensitivity and specificity, no STRATIFICATION risk, low cost). Pulmonary angiography is regarded as the The factors predisposing to VTE broadly fit Virchow’s triad of venous stasis, injury to the final arbiter but is ill suited for diagnosing a disease vein wall, and enhanced coagulability of the present in only a third of patients in whom it is suspected. blood (box 1). The identification of risk factors Some tests are good for confirmation and some for aids clinical diagnosis of VTE and guides decisions about repeat testing in borderline exclusion of embolism; others are able to do both but are cases. Primary ‘‘thrombophilic’’ abnormalities often non-diagnostic. For optimal efficiency, choice of the need to interact with acquired risk factors before initial test should be guided by clinical assessment of the thrombosis occurs; they are usually discovered after the thromboembolic event. Therefore, the likelihood of embolism and by patient characteristics that risk of VTE is best assessed by recognising the may influence test accuracy. Standardised clinical presence of known ‘‘clinical’’ risk factors. estimates can be used to give a pre-test probability to However, investigations for thrombophilic dis- orders at follow up should be considered in those assess, after appropriate objective testing, the post-test without another apparent explanation. -

Chest and Abdominal Radiograph 101
Chest and Abdominal Radiograph 101 Ketsia Pierre MD, MSCI July 16, 2010 Objectives • Chest radiograph – Approach to interpreting chest films – Lines/tubes – Pneumothorax/pneumomediastinum/pneumopericar dium – Pleural effusion – Pulmonary edema • Abdominal radiograph – Tubes – Bowel gas pattern • Ileus • Bowel obstruction – Pneumoperitoneum First things first • Turn off stray lights, optimize room lighting • Patient Data – Correct patient – Patient history – Look at old films • Routine Technique: AP/PA, exposure, rotation, supine or erect Approach to Reading a Chest Film • Identify tubes and lines • Airway: trachea midline or deviated, caliber change, bronchial cut off • Cardiac silhouette: Normal/enlarged • Mediastinum • Lungs: volumes, abnormal opacity or lucency • Pulmonary vessels • Hila: masses, lymphadenopathy • Pleura: effusion, thickening, calcification • Bones/soft tissues (four corners) Anatomy of a PA Chest Film TUBES Endotracheal Tubes Ideal location for ETT Is 5 +/‐ 2 cm from carina ‐Normal ETT excursion with flexion and extension of neck 2 cm. ETT at carina Right mainstem Intubation ‐Right mainstem intubation with left basilar atelectasis. ETT too high Other tubes to consider DHT down right mainstem DHT down left mainstem NGT with tip at GE junction CENTRAL LINES Central Venous Line Ideal location for tip of central venous line is within superior vena cava. ‐ Risk of thrombosis decreased in central veins. ‐ Catheter position within atrium increases risk of perforation Acceptable central line positions • Zone A –distal SVC/superior atriocaval junction. • Zone B – proximal SVC • Zone C –left brachiocephalic vein. Right subclavian central venous catheter directed cephalad into IJ Where is this tip? Hemiazygous Or this one? Right vertebral artery Pulmonary Arterial Catheter Ideal location for tip of PA catheter within mediastinal shadow. -

Cardiothoracic Fellowship Program
Cardiothoracic Fellowship Program Table of Contents Program Contact ............................................................................................ 3 Other contact numbers .................................................................................. 4 Introduction ........................................................................................................... 5 Goals and Objectives of Fellowship: ..................................................................... 6 Rotation Schedule: ........................................................................................ 7 Core Curriculum .................................................................................................... 8 Fellow’s Responsibilities ..................................................................................... 22 Resources ........................................................................................................... 23 Facilities ....................................................................................................... 23 Educational Program .......................................................................................... 26 Duty Hours .......................................................................................................... 29 Evaluation ........................................................................................................... 30 Table of Appendices .................................................................................... 31 Appendix A -

Mantke, Peitz, Surgical Ultrasound -- Index
419 Index A esophageal 218 Anorchidism 376 gallbladder 165 Aorta 364–366 A-mode imaging 97 gastric 220 abdominal aneurysm (AAA) AAA (abdominal aortic aneurysm) metastasis 142 20–21, 364, 366 20–21, 364, 366 pancreatic 149, 225 dissection 364, 366 Abdominal wall Adenofibroma, breast 263 perforation 366 abscess 300–301 Adenoma pseudoaneurysm 364 diagnostic evaluation 297 adrenal 214 Aortic rupture 20 hematoma 73, 300, 305 colorectal 231, 232 Aplasia, muscular 272 rectus sheath 297–300 duodenal papilla 229, 231 Appendicitis 1–4 hernia 300, 302–304 gallbladder 165 consequences for surgical indications for sonography 297 hepatic 54, 58, 141 treatment 2 seroma 298, 300, 305 multiple 141 sonographic criteria 1 trauma 297–300 parathyroid 213 Archiving 418 Abortion, tubal 30 renal 241 Arteriosclerosis 346, 348 Abscess thyroid 202–203 carotid artery 335, 337, 338 abdominal wall 300–301 Adenomyomatosis 8, 164, 165 plaque 337, 338, 345, 367, 370 causes 301 Adrenal glands 214–216 Arteriovenous (AV) malformation amebic 138 adenoma 214 139, 293, 326–329 breast 264 carcinoma 214 Artery chest wall 173, 178 cyst 214 carotid 334–339 diverticular 120, 123 hematoma 214 aneurysm 338 drainage 85–88, 93 hemorrhage 214 arteriosclerosis 335 hepatic 6, 138, 398 hyperplasia 214 plaque characteristics inflammatory bowel disease limpoma/myelipoma 214 337, 338, 345 116, 119 metastases 214 bifurcation 334, 337 intramural 5 sonographic criteria 214 bulb 339 lung 183, 186, 190 tuberculosis 214 dissection 338, 339, 346 pancreatic 11 Advanced dynamic flow (ADF) sonographic -

The Supine Pneumothorax
Annals of the Royal College of Surgeons of England (1987) vol. 69 The supine pneumothorax DAVID A P COOKE FRCS Surgical Registrar, Department ofSurgery, St Thomas' Hospital JULIE C COOKE FRCR* Radiological Senior Registrar, Department ofDiagnostic Radiology, Brompton Hospital, London Key words: PNEUMOTHORAX; COMPUTI ED TOMOGRAPHY; TRAUMA Summary TABLE I Causes of a pneumothorax The consequences of an undiagnosed pneumothorax can be life- threatening, particularly in patients with trauma to the head or Broncho-pulmonay pathology Traumatic injuy and in those mechanical ventilation. Yet multiple requiring Asthma it is these patients, whose films will be assessed initially by the Bronchial adenoma surgeon, who are more likely to have a chest X-ray taken in the Bronchial carcinoma Penetrating trauma supine position. The features of supine pneumothoraces are de- Emphysema Blunt trauma scribed and discussed together with radiological techniques used to Fibrosing alveolitis Inhaled foreign body confirm the diagnosis, including computed tomography (CT) Idiopathic which may be ofparticular importance in patients with associated Marfan's syndrome fatrogenic cranial trauma. Pulmonary abscess Pulmonary dysplasia CVP line insertion Introduction Pulmonary infarct Jet ventilation Pulmonary metastases Liver biopsy In a seriously ill patient or the victim of multiple trauma Pulmonoalveolar proteinosis Lung biopsy the clinical symptoms and signs of a pneumothorax may Radiation pneumonitis Oesophageal instrumentation be overshadowed by other problems. Usually in these Sarcoid PEEP ventilation circumstances a chest X-ray will be taken at the bedside Staphylococcal septicaemia Pleural aspiration with the patient supine and the appearances of a Tuberculosis Pleural biopsy pneumothorax will be different from those seen when the Tuberose sclerosis patient is upright. -

Toxicological Profile for Jp-5, Jp-8, and Jet a Fuels
TOXICOLOGICAL PROFILE FOR JP-5, JP-8, AND JET A FUELS U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry March 2017 JP-5, JP-8, AND JET A FUELS ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. JP-5, JP-8, AND JET A FUELS iii UPDATE STATEMENT A Toxicological Profile for JP-5, JP-8, and Jet A Fuels, Draft for Public Comment was released in February 2016. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Human Health Sciences Environmental Toxicology Branch 1600 Clifton Road NE Mailstop F-57 Atlanta, Georgia 30329-4027 JP-5, JP-8, AND JET A FUELS iv This page is intentionally blank. JP-5, JP-8, AND JET A FUELS v FOREWORD This toxicological profile is prepared in accordance with guidelines* developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. -

12. Shortness of Breath
12. Shortness of breath Shortness of breath (dyspnea) is described as an intense tightening in the chest, air hunger, difficulty breathing, breathlessness or a feeling of suffocation. It is a subjective symptom of many diseases. The causes of shortness of breath can be various, but most often pulmonary or cardiac. The main causes of pulmonary or cardiac dyspnea include heart congestion, pulmonary embolism, emphysema, atelectasis, pneumothorax and interstitial lung processes. The basic imaging method in the diagnosis of dyspnea that follows clinical examination is a chest X- ray. It is used to evaluate heart congestion, fluid effusions, atelectasis, pneumothorax, and a rough assessment of interstitial lung processes. Only if there is a clinical suspicion of pulmonary embolization, the CT angiography (with intravenous contrast), perfusion lung scintigraphy or echocardiography are imaging methods of choice. If interstitial lung disease or pulmonary emphysema are suspected or there is uncertain finding on chest X-ray, high-resolution CT (HRCT) of the chest is recommended. HRCT allows detailed assessment of pulmonary parenchyma. Non-contrast CT is usually sufficient to assess interstitial changes. Heart congestion Heart congestion is the result of insufficient cardiac output due to heart failure or fluid overload. The most common cause of heart congestion is left heart failure with increased pressure in pulmonary veins and capillaries. On chest X- ray can be distinguished 3 degrees of heart congestion. Note: Right-sided heart failure most often occurs in long-term left-sided heart failure or lung disease, which leads to high pulmonary artery resistance (various etiologies of pulmonary hypertension, e.g. advanced COPD). -

Interstitial Lung Disease
Interstitial Lung Disease Camille Washowich, MSN, ACNP, CCRN Center for Advanced Lung Disease Stanford University Medical Center Lung Physiology ILD Classification Interstitial Lung Disease Connective Tissue Diseases Primary (unclassified) Idiopathic Fibrotic Disorders Drug and Treatment Induced Connective Tissue Diseases Scleroderma Systemic Lupus Erythematous (SLE) Rheumatoid Arthritis Mixed Connective Tissue Disease Primary (unclassified) Sarcoidosis Stage I-IV Neurofibromatosis Tuberous Sclerosis AIDS ARDS Bone Marrow Transplantation Post infectious Occupational & Environmental Exposures: Inorganic & Organic Agriculture Workers and Animal Handlers Construction: wood/metal Auto repair Military Chemicals (plastic, paint, polyurethane) Organisms: fungus/molds/bacterium Idiopathic Fibrotic Disorders Pulmonary fibrosis Familial pulmonary fibrosis Autoimmune pulmonary fibrosis Respiratory bronchiolitis Nonspecific interstitial pneumonitis (NSIP) Drug Induced Antibiotics Anti-arrhythmics Anti-inflammatory Anti-convulsant Radiation/Chemotherapy Oxygen toxicity Narcotics ILD Epidemiology in the US 100K admissions/year Occupation DILD Sarcoidosis 11% 15% pulmonologist patients 5% DAH 8% 4% CTD Incidence: 5/100K 9% Men (31%) versus Women (26%) Other 11% IPF 45% of all ILD patients Pulmonary Fibrosis 52% Age/Gender/Race Specifications to Assist in Diagnosis 20-40yrs: Inherited Interstitial Lung diseases Familial idiopathic pulmonary fibrosis Collagen vascular disease- associated ILD LAM Pulmonary Langerhans’ cell granulomatosis Sarcoidosis 50yrs: -
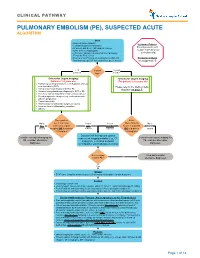
Pulmonary Embolism (Pe), Suspected Acute Algorithm
CLINICAL PATHWAY PULMONARY EMBOLISM (PE), SUSPECTED ACUTE ALGORITHM Start · Room air pulse oximetry Inclusion Criteria: · Consider supplemental oxygen · IV access and STAT CBC and DIC screen Patient presents with · Urine β-HCG, if appropriate suspected Pulmonary · CXR (PA + lateral), EKG and call the Cardiology Embolism (PE) Fellow for Echo review · Review criteria* for urgent imaging for possible PE Exclusion Criteria based on age-specific risk factors (see green boxes) No suspected PE < 18 Patient 18 years years old age? or older *Criteria for Urgent Imaging: *Criteria for Urgent Imaging: Patients < 18 years old For patients ≥ 18 years old · Painful leg swelling or known recent diagnosis of deep vein thrombosis (DVT) Please refer to the Wells Criteria · Family or personal history of DVT or PE Algorithm on page 7. · Known clotting disorder predisposing to DVT or PE · Recent or current indwelling central venous catheter · Elevated systemic estrogen (e.g., oral contraceptive pill use, pregnancy) · Recent immobility · Recent major or orthopedic surgery or trauma · Acute or chronic inflammatory condition · Obesity Does patient Is the No to meet 1 or more Yes to Yes to Wells Criteria No to ALL criteria for urgent ANY EITHER Score > 4 points BOTH criteria imaging OR is d-dimer criteria criterion OR is d-dimer criteria > 0.5ug/mL? > 0.5ug/mL? Discuss CXR findings and optimal Consider non-urgent imaging for subsequent imaging modality (e.g., CT Consider non-urgent imaging for PE, consider alternative angiogram, ventilation perfusion PE, consider -

CHEST RADIOLOGY: Goals and Objectives
Harlem Hospital Center Department of Radiology Residency Training Program CHEST RADIOLOGY: Goals and Objectives ROTATION 1 (Radiology Years 1): Resident responsibilities: • ED chest CTs • Inpatient and outpatient plain films including the portable intensive care unit radiographs • Consultations with referring clinicians MEDICAL KNOWLEDGE: • Residents must demonstrate knowledge about established and evolving biomedical, clinical, and cognitive sciences and the application of this knowledge to patient care. At the end of the rotation, the resident should be able to: • Identify normal radiographic and CT anatomy of the chest • Identify and describe common variants of normal, including aging changes. • Demonstrate a basic knowledge of radiographic interpretation of atelectasis, pulmonary infection, congestive heart failure, pleural effusion and common neoplastic diseases of the chest • Identify the common radiologic manifestation of thoracic trauma, including widened mediastinum, signs of aortic laceration, pulmonary contusion/laceration, esophageal and diaphragmatic rupture. • Know the expected postoperative appearance in patients s/p thoracic surgery and the expected location of the life support and monitoring devices on chest radiographs of critically ill patients (intensive care radiology); be able to recognize malpositioned devices. • Identify cardiac enlargement and know the radiographic appearance of the dilated right vs. left atria and right vs. left ventricles, and pulmonary vascular congestion • Recognize common life-threatening