Interstitial Lung Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
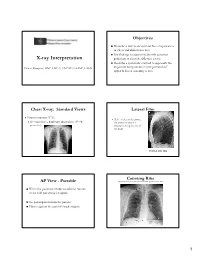
X-Ray Interpretation
Objectives Describe a systematic method for interpretation of chest and abdomen x-rays List findings to accurately identify common X-ray Interpretation pathology in chest & abdomen x-rays Describe a systematic method to approach the Denise Ramponi, DNP, FNP-C, ENP-BC, FAANP, FAEN important components in interpretation of upper & lower extremity x-rays Chest X-ray: Standard Views Lateral Film Postero-anterior (PA): (LAT) view can determine th On inspiration – diaphragm descends to 10 rib the anterior-posterior posteriorly structures along the axis of the body Normal LAT film Counting Ribs AP View - Portable http://www.lumen.luc.edu/lumen/MedEd/medicine/pulmonar/cxr/cxr_f.htm When the patient is unable to tolerate routine views with pts sitting or supine No participation from the patient Film is against the patient's back (supine) 1 Consolidation, Atelectasis, Chest radiograph Interstitial involvement Consolidation - any pathologic process that fills the alveoli with Left and right heart fluid, pus, blood, cells or other borders well defined substances Interstitial - involvement of the Both hemidiaphragms supporting tissue of the lung visible to midline parenchyma resulting in fine or coarse reticular opacities Right - higher Atelectasis - collapse of a part of Heart less than 50% of the lung due to a decrease in the amount of air resulting in volume diameter of the chest loss and increased density. Infiltrate, Consolidation vs. Congestive Heart Failure Atelectasis Fluid leaking into interstitium Kerley B 2 Kerley B lines Prominent interstitial markings Kerley lines Magnified CXR Cardiomyopathy & interstitial pulmonary edema Short 1-2 cm white lines at lung periphery horizontal to pleural surface Distended interlobular septa - secondary to interstitial edema. -

Cardiothoracic Fellowship Program
Cardiothoracic Fellowship Program Table of Contents Program Contact ............................................................................................ 3 Other contact numbers .................................................................................. 4 Introduction ........................................................................................................... 5 Goals and Objectives of Fellowship: ..................................................................... 6 Rotation Schedule: ........................................................................................ 7 Core Curriculum .................................................................................................... 8 Fellow’s Responsibilities ..................................................................................... 22 Resources ........................................................................................................... 23 Facilities ....................................................................................................... 23 Educational Program .......................................................................................... 26 Duty Hours .......................................................................................................... 29 Evaluation ........................................................................................................... 30 Table of Appendices .................................................................................... 31 Appendix A -

12. Shortness of Breath
12. Shortness of breath Shortness of breath (dyspnea) is described as an intense tightening in the chest, air hunger, difficulty breathing, breathlessness or a feeling of suffocation. It is a subjective symptom of many diseases. The causes of shortness of breath can be various, but most often pulmonary or cardiac. The main causes of pulmonary or cardiac dyspnea include heart congestion, pulmonary embolism, emphysema, atelectasis, pneumothorax and interstitial lung processes. The basic imaging method in the diagnosis of dyspnea that follows clinical examination is a chest X- ray. It is used to evaluate heart congestion, fluid effusions, atelectasis, pneumothorax, and a rough assessment of interstitial lung processes. Only if there is a clinical suspicion of pulmonary embolization, the CT angiography (with intravenous contrast), perfusion lung scintigraphy or echocardiography are imaging methods of choice. If interstitial lung disease or pulmonary emphysema are suspected or there is uncertain finding on chest X-ray, high-resolution CT (HRCT) of the chest is recommended. HRCT allows detailed assessment of pulmonary parenchyma. Non-contrast CT is usually sufficient to assess interstitial changes. Heart congestion Heart congestion is the result of insufficient cardiac output due to heart failure or fluid overload. The most common cause of heart congestion is left heart failure with increased pressure in pulmonary veins and capillaries. On chest X- ray can be distinguished 3 degrees of heart congestion. Note: Right-sided heart failure most often occurs in long-term left-sided heart failure or lung disease, which leads to high pulmonary artery resistance (various etiologies of pulmonary hypertension, e.g. advanced COPD). -

Chest Radiology: a Resident's Manual
Chest Radiology: A Resident's Manual Bearbeitet von Johannes Kirchner 1. Auflage 2011. Buch. 300 S. Hardcover ISBN 978 3 13 153871 0 Format (B x L): 23 x 31 cm Weitere Fachgebiete > Medizin > Sonstige Medizinische Fachgebiete > Radiologie, Bildgebende Verfahren Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. 1 Heart Failure Acute left heart failure is most commonly caused by a hyperten- " Compare pulmonary vessels that are equidistant to a central sive crisis. Radiographic signs on the plain chest radiograph ob- point in the respective hilum. tained with the patient standing include: " Compare the diameter of a random easily identifiable superior " Redistribution of pulmonary perfusion lobe artery (often the anterior segmental artery is most easily " Presence of interstitial patterns (Kerley lines, peribronchial identifiable) with the diameter of the corresponding ipsilateral cuffing) bronchus (Fig. 1.62). " Alveolar densities with indistinct vascular structures (ad- vanced stage) As the pulmonary artery and corresponding ipsilateral bronchus " Pleural effusions are normally of precisely equal diameter, a larger arterial diameter is indicative of redistribution of perfusion (Fig. 1.63). The diagnos- All of these signs are essentially attributable to increased fluid tic criteria of caudal-to-cranial redistribution cannot be evaluated content in the abnormally heavy “wet” lung. The fluid accumula- on radiographs obtained in the supine patient. -

Pleural Effusion, Hypovascularity in Lung Zone (Westermark’S Sign) & Pyramid Shape Infiltrate with Peak Directed to Hilus (Hampton’S Hump)
Author(s): Michele M. Nypaver, MD, 2011 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/ We have reviewed this material in accordance with U.S. Copyright Law and have tried to maximize your ability to use, share, and adapt it. Copyright holders of content included in this material should contact [email protected] with any questions, corrections, or clarification regarding the use of content. For more information about how to cite these materials visit http://open.umich.edu/privacy-and-terms-use. Any medical information in this material is intended to inform and educate and is not a tool for self-diagnosis or a replacement for medical evaluation, advice, diagnosis or treatment by a healthcare professional. Please speak to your physician if you have questions about your medical condition. Viewer discretion is advised: Some medical content is graphic and may not be suitable for all viewers. Citation Key for more information see: http://open.umich.edu/wiki/CitationPolicy Use + Share + Adapt { Content the copyright holder, author, or law permits you to use, share and adapt. } Public Domain – Government: Works that are produced by the U.S. Government. (17 USC § 105) Public Domain – Expired: Works that are no longer protected due to an expired copyright term. Public Domain – Self Dedicated: Works that a copyright holder has dedicated to the public domain. Creative Commons – Zero Waiver Creative Commons – Attribution License Creative Commons – Attribution Share Alike License Creative Commons – Attribution Noncommercial License Creative Commons – Attribution Noncommercial Share Alike License GNU – Free Documentation License Make Your Own Assessment { Content Open.Michigan believes can be used, shared, and adapted because it is ineligible for copyright. -

Eponyms in Radiologic Signs
Eponyms in radiologic signs Poster No.: C-0133 Congress: ECR 2014 Type: Educational Exhibit Authors: D. Andrade, L. Andrade, M. Magalhaes, L. Curvo-Semedo, F. Caseiro Alves; Coimbra/PT Keywords: Diagnostic procedure, Fluoroscopy, CT, Conventional radiography, Thorax, Musculoskeletal system, Gastrointestinal tract, Education and training DOI: 10.1594/ecr2014/C-0133 Any information contained in this pdf file is automatically generated from digital material submitted to EPOS by third parties in the form of scientific presentations. References to any names, marks, products, or services of third parties or hypertext links to third- party sites or information are provided solely as a convenience to you and do not in any way constitute or imply ECR's endorsement, sponsorship or recommendation of the third party, information, product or service. ECR is not responsible for the content of these pages and does not make any representations regarding the content or accuracy of material in this file. As per copyright regulations, any unauthorised use of the material or parts thereof as well as commercial reproduction or multiple distribution by any traditional or electronically based reproduction/publication method ist strictly prohibited. You agree to defend, indemnify, and hold ECR harmless from and against any and all claims, damages, costs, and expenses, including attorneys' fees, arising from or related to your use of these pages. Please note: Links to movies, ppt slideshows and any other multimedia files are not available in the pdf version of presentations. www.myESR.org Page 1 of 43 Learning objectives 1. To recognize the most frequent and important radiologic signs that are eponyms. -

A Rare Case of Isolated Chronic Cough Caused by Pulmonary Lymphangitic Carcinomatosis As a Primary Manifestation of Rectum Carcinoma
doi: 10.2169/internalmedicine.0572-17 Intern Med Advance Publication http://internmed.jp 【 CASE REPORT 】 A Rare Case of Isolated Chronic Cough caused by Pulmonary Lymphangitic Carcinomatosis as a Primary Manifestation of Rectum Carcinoma Minami Okayama, Yoshihiro Kanemitsu, Tetsuya Oguri, Takamitsu Asano, Satoshi Fukuda, Hirotsugu Ohkubo, Masaya Takemura, Ken Maeno, Yutaka Ito and Akio Niimi Abstract: A 36-year old man was referred to our hospital due to isolated chronic cough that was refractory to anti- asthma medications, including inhaled corticosteroids/long-acting β2 agonists. Chest X-ray showed diffuse nodular and enhanced vascular shadows with Kerley lines in both lungs. A blood analysis showed elevated serum CEA and CA19-9 levels. A transbronchial biopsy revealed well to moderately differentiated adenocar- cinoma, the origin of which was immunohistochemically suspected to be the gastrointestinal tract. Colono- scopy confirmed the diagnosis of primary rectum carcinoma. Pulmonary lymphangitic carcinomatosis was therefore regarded as the origin of the cough. Lymphangitic carcinomatosis is an uncommon diagnosis but important to consider in patients with persistent cough. Key words: chronic cough, pulmonary lymphangitic carcinomatosis, rectum carcinoma (Intern Med Advance Publication) (DOI: 10.2169/internalmedicine.0572-17) leukotriene antagonist under a tentative diagnosis of cough- Introduction variant asthma; however, the cough was refractory to such intensive anti-asthma therapies. Although cough is one of the most common symptoms of At the first visit, he complained of only a persistent lung cancer (1), the frequency of lung cancer as a cause of cough, with no other symptoms, such as a fever, dyspnea, chronic cough as the sole symptom is less than 2% (2). -

Approach to Respiratory Failure in Emergency Department
European Review for Medical and Pharmacological Sciences 2006; 10: 135-151 Approach to respiratory failure in emergency department P. FORTE, M. MAZZONE, G. PORTALE, C. FALCONE, F. MANCINI, N. GENTILONI SILVERI Department of Emergency Medicine, Catholic University – Rome (Italy) Abstract. – Objectives and Background: The second mechanism (mismatch in blood The goal of this review is to provide update rec- gas exchanges) is due to the following differ- ommendations that can be used by emergency ent pathologies: physicians who provide primary cares to pa- tients with Acute Respiratory Failure (ARF), from • Adult acute respiratory distress syn- the admission to an emergency department drome; through the first 24 to 48 hours of hospitaliza- • Neonatal respiratory distress syndrome; tion. This work wants to address the diagnosis • Acute cardiogenic pulmonary oedema; and emergency medical care of ARF and the • Severe status asthmaticus; management of medical complications. • Pneumonia; State of the Art: A lot of statement has been developed for the early management and treat- • Airspace collapse (atelectasis); ment of ARF; moreover, over the last fifteen • Pulmonary embolism. years, we have assisted to the rise of a new technique of ventilation, in the Emergency De- The clinical signs and symptoms of patients partment: Non Invasive Ventilation. This kind of with ARF, refer to the two main manifesta- ventilation was firsthy applied in Intensive Care tions of pulmonary diseases: arterial hyper- and in Respiratory Care Unit. Randomized con- capnia and hypoxemia. trolled clinical trials have showed its usefulness in the early treatment of several forms of ARF, The pathophysiology of hypercapnia is together with medical therapy. -

CT Images After Lung Transplantation
CT images after Lung Transplantation Kikuchi N.,M.D., Honda O.,M.D.,Ph.D., Hata A,M.D., Yanagawa M.,M.D.,Ph.D., Tomiyama N.,M.D.,Ph.D . Department of Radiology Osaka University Graduate School of Medicine,Osaka,Japan No financial relationship to disclose. Introduction Nearly 50,000 lung transplantation have been performed until 2013 in the world. In our hospital, 51cases have undergone lung transplantation during 2000-2016. Complications may follow after lung a transplantation o early(~1week) o intermediate(1week~2months) o late(2months~) And we also show radiographs cases that we have experienced. Early Posttransplant Period(~1week) Surgical complications Airspace&interstitial disease Pulmonary vein thrombosis Hyperacute rejection Pulmonary thromboembolism Graft size mismatch Phrenic nerve paralysis Primary graft dysfunction Pleural complications Lober torsion Pleural effusion Pulmonary edema Pneumothorax Hemothorax Surgical complications Pulmonary vein thrombosis Thrombi are formed at the pulmonary venous/left atrial anatomic suture line. They may carry the risk of systemic embolization and cerebrovascular accident, and obstruct pulmonary venous outflow and cause severe pulmonary edema. Clinical features include hypoxemia, decreased lung compliance, and diffuse radiographic opacities in the allograft. But some cases were detected incidentally by transesophageal echocardiography without any symptoms. Uhlmann EJ et al:J Heart Lung Transplant 2009; 28:209. Surgical complications Pulmonary thromboembolism(PE) PE can occur at any time after lung transplantation but is most common in the early period. Postoperative immobility and hypercoagulability are important risk factor. Imaging findings On CXR, Westermark sign(focal peripheral hyperlucency and central pulmonary vessels may also be dilated), secondary findings of infraction: Hampton hump sign(a dome shaped, pleural-based opacification) and unilateral pulmonary oligemia are seen. -

High Altitude Medical Problems (Medi- Medical Progress Cal Progress)
Refer to: Hultgren HN: High altitude medical problems (Medi- Medical Progress cal Progress). West J Med 131:8-23, Jul 1979 High Altitude Medical Problems HERBERT N. HULTGREN, MD, Palo Alto, California Increased travel to high altitude areas by mountaineers and nonclimbing tour- ists has emphasized the clinical problems associated with rapid ascent. Acute mountain sickness affects most sojourners at elevations above 10,000 feet. Symptoms are usually worse on the second or third day after arrival. Gradual ascent, spending one to three days at an intermediate altitude, and the use of acetazolamide (Diamox) will prevent or ameliorate symptoms in most instances. Serious and potentially fatal problems, such as high altitude pulmonary edema or cerebral edema, occur in approximately 0.5 percent to 1.0 percent of visitors to elevations above 10,000 feet-especially with heavy physical exertion on arrival, such as climbing or skiing. Early recognition, high flow oxygen therapy and prompt descent are crucially important in management. Our knowledge of the causes of these and other high altitude problems, such as retinal hemor- rhage, systemic edema and pulmonary hypertension, is still incomplete. Even less is known of the effect of high altitudes on medical conditions common at sea level or on the action of commonly used drugs. EXPOSURE TO HIGH ALTITUDE of people of all hiked up to Pheriche (14,000 feet) en route to the ages and degrees of health has enormously in- Everest base camp. In the Mount Kenya area 3,- creased in the last ten years. Modern travel facili- 500 climbers and trekkers climb above 13,000 ties and the advent of mountain tours and trek- feet each year. -
Pleural Effusion
1 เอกสารประกอบการสอน เรื่อง รังสีวิทยาระบบทางเดินหายใจ: การเลือกส่งตรวจและแปลผลภาพรังสีทรวงอก (Radiology of the chest: methods of investigation and plain film interpretation) โดย แพทย์หญิงวรรณพร บุรีวงษ์ 2 แผนการสอน หัวข้อ รังสีวิทยาระบบทางเดินหายใจ: การเลือกส่งตรวจและแปลผลภาพรังสีทรวงอก ผู้สอน พญ. วรรณพร บุรีวงษ์ เวลา 3 ชั่วโมง วัตถุประสงค์ เพื่อให้นิสิตแพทย์สามารถ 1. บอกวิธีการตรวจและข้อบ่งชี้ในการส่งตรวจทางรังสีวิทยาของระบบทางเดินหายใจได้ 2. บอกท่าที่ใช้ถ่ายภาพรังสีทรวงอก และความเหมาะสมของเทคนิคที่ใช้ในการถ่ายภาพได้ 3. สามารถอธิบายโครงสร้างและอวัยวะภายในของร่างกายที่พบบนภาพรังสีทรวงอกได้ เนื้อหาหัวข้อ 1. วิธีการตรวจทางรังสีวิทยาของระบบทางเดินหายใจ ได้แก่ Plain chest radiography (CXR), Computed tomography (CT), CT angiography, Magnetic resonance imaging (MRI), Ultrasonography, Angiography และ Radionuclide study 2. ลักษณะทางกายวิภาคเบื้องต้นของระบบทางเดินหายใจ (Normal anatomy of the chest) 3. ภาพถ่ายรังสีทรวงอกแบบปกติ (Normal chest radiography) การจัดประสบการณ์เรียนรู้ 1. บอกวัตถุประสงค์และบอกเนื้อหา 5 นาที 2. สอนบรรยายหัวข้อต่างๆ 60 นาที 3. กิจกรรม/สอนแสดง 90 นาที 4. สรุปเนื้อหาบทเรียน 15 นาที 5. นิสิตซักถาม 10 นาที สื่อการสอน 1. เอกสารประกอบการสอน 2. Power point ทั้งภาพนิ่งและ animation 3. ภาพถ่ายทางรังสี วิธีประเมินผล 1. ข้อสอบ Multiple choice question 5 ตัวเลือก 2. ข้อสอบบรรยายภาพถ่ายทางรังสี OSCE 3 หนังสือและเอกสารอ้างอิง 1. Sutton D. Textbook of radiology and imaging. 6th ed. China: Churchill Living stone, 1998 2. Herring W. Learning radiology: recognizing the basics. 2nd ed. Philadelphia: Elsevier Mosby, 2007. 3. Armstrong P, -
Chest Imaging
ISSN: 2165-3259 JAOCR Official Journal of the American Osteopathic College of Radiology CHEST IMAGING Guest Editors: Les Folio, D.O., MPH, FAOCR Bernard F. Laya, D.O. Editor-in Chief: William T. O’Brien, Sr., D.O. April 2014, Vol. 3, Issue 2 JAOCR About the Journal Aims and Scope The Journal of the American Osteopathic College of Radiology (JAOCR) is designed to provide practical up-to- date reviews of critical topics in radiology for practicing radiologists and radiology trainees. Each quarterly issue covers a particular radiology subspecialty and is composed of high quality review articles and case reports that highlight differential diagnoses and important teaching points. Access to Articles All articles published in the JAOCR are open access online. Subscriptions to the journal are not required to view or download articles. Reprints are not available. Copyrights Materials published in the JAOCR are protected by copyright. No part of this publication may be reproduced without written permission from the AOCR. Guide for Authors Submissions for the JAOCR are by invitation only. If you were invited to submit an article and have questions regarding the content or format, please contact the appropriate Guest Editor for that particular issue. Although contributions are invited, they are subject to peer review and final acceptance. Editor-in-Chief William T. O’Brien, Sr., D.O. San Antonio, TX Design Editor Jessica Roberts Communications Director, AOCR Managing Editor Tammam Beydoun, D.O. Farmington Hills, MI Editorial Board Susann Schetter, D.O. Daniel J. Abbis, D.O. Les R. Folio, D.O. Michael W. Keleher, D.O.