1 Merck &21: Disorders Due to Physical Agents Chapter 253
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevention and Management of Heat-Related Illness
PREVENTION AND MANAGEMENT OF HEAT-RELATED ILLNESS Federal Bureau of Prisons Clinical Guidance DECEMBER 2017 Federal Bureau of Prisons (BOP) Clinical Guidance is made available to the public for informational purposes only. The BOP does not warrant this guidance for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient- specific. Consult the BOP Health Management Resources Web page to determine the date of the most recent update to this document: http://www.bop.gov/resources/health_care_mngmt.jsp Federal Bureau of Prisons Prevention and Management of Heat-Related Illness Clinical Guidance December 2017 TABLE OF CONTENTS 1. PURPOSE AND OVERVIEW ......................................................................................................................1 2. PATHOPHYSIOLOGY...............................................................................................................................1 3. RISK FACTORS FOR HRI ........................................................................................................................2 4. SYMPTOMS AND SIGNS ..........................................................................................................................3 5. EVALUATION.........................................................................................................................................5 6. TREATMENT..........................................................................................................................................6 -
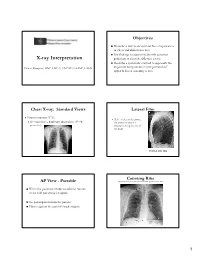
X-Ray Interpretation
Objectives Describe a systematic method for interpretation of chest and abdomen x-rays List findings to accurately identify common X-ray Interpretation pathology in chest & abdomen x-rays Describe a systematic method to approach the Denise Ramponi, DNP, FNP-C, ENP-BC, FAANP, FAEN important components in interpretation of upper & lower extremity x-rays Chest X-ray: Standard Views Lateral Film Postero-anterior (PA): (LAT) view can determine th On inspiration – diaphragm descends to 10 rib the anterior-posterior posteriorly structures along the axis of the body Normal LAT film Counting Ribs AP View - Portable http://www.lumen.luc.edu/lumen/MedEd/medicine/pulmonar/cxr/cxr_f.htm When the patient is unable to tolerate routine views with pts sitting or supine No participation from the patient Film is against the patient's back (supine) 1 Consolidation, Atelectasis, Chest radiograph Interstitial involvement Consolidation - any pathologic process that fills the alveoli with Left and right heart fluid, pus, blood, cells or other borders well defined substances Interstitial - involvement of the Both hemidiaphragms supporting tissue of the lung visible to midline parenchyma resulting in fine or coarse reticular opacities Right - higher Atelectasis - collapse of a part of Heart less than 50% of the lung due to a decrease in the amount of air resulting in volume diameter of the chest loss and increased density. Infiltrate, Consolidation vs. Congestive Heart Failure Atelectasis Fluid leaking into interstitium Kerley B 2 Kerley B lines Prominent interstitial markings Kerley lines Magnified CXR Cardiomyopathy & interstitial pulmonary edema Short 1-2 cm white lines at lung periphery horizontal to pleural surface Distended interlobular septa - secondary to interstitial edema. -

Cardiothoracic Fellowship Program
Cardiothoracic Fellowship Program Table of Contents Program Contact ............................................................................................ 3 Other contact numbers .................................................................................. 4 Introduction ........................................................................................................... 5 Goals and Objectives of Fellowship: ..................................................................... 6 Rotation Schedule: ........................................................................................ 7 Core Curriculum .................................................................................................... 8 Fellow’s Responsibilities ..................................................................................... 22 Resources ........................................................................................................... 23 Facilities ....................................................................................................... 23 Educational Program .......................................................................................... 26 Duty Hours .......................................................................................................... 29 Evaluation ........................................................................................................... 30 Table of Appendices .................................................................................... 31 Appendix A -

HHE Report No. HETA-2018-0154-3361, Evaluation of Rhabdomyolysis and Heat Stroke in Structural Firefighter Cadets
Evaluation of Rhabdomyolysis and Heat Stroke in Structural Firefighter Cadets HHE Report No. 2018-0154-3361 November 2019 Authors: Judith Eisenberg, MD, MS Jessica F. Li, MSPH Karl D. Feldmann, MS, CIH Desktop Publisher: Jennifer Tyrawski Editor: Cheryl Hamilton Logistics: Donnie Booher, Kevin Moore Medical Field Assistance: Nonita Dhirar Data Support: Hannah Echt Keywords: North American Industry Classification System (NAICS) 922160 (Fire Protection), Structural Firefighter Training, Structural Firefighter Cadet Course, Heat, Heat-Related Illness, Heat Stroke, Rhabdomyolysis, Texas Disclaimer The Health Hazard Evaluation Program investigates possible health hazards in the workplace under the authority of the Occupational Safety and Health Act of 1970 [29 USC 669a(6)]. The Health Hazard Evaluation Program also provides, upon request, technical assistance to federal, state, and local agencies to investigate occupational health hazards and to prevent occupational disease or injury. Regulations guiding the Program can be found in Title 42, Code of Federal Regulations, Part 85; Requests for Health Hazard Evaluations [42 CFR Part 85]. Availability of Report Copies of this report have been sent to the employer, employees, and union at the plant. The state and local health departments and the Occupational Safety and Health Administration Regional Office have also received a copy. This report is not copyrighted and may be freely reproduced. Recommended Citation NIOSH [2019]. Evaluation of rhabdomyolysis and heat stroke in structural firefighter cadets. By Eisenberg J, Li JF, Feldmann KD. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Health Hazard Evaluation Report 2018-0154-3361, https://www.cdc.gov/niosh/hhe/reports/pdfs/2018-0154-3361.pdf. -

Occupational Exposure to Heat and Hot Environments
Criteria for a Recommended Standard Occupational Exposure to Heat and Hot Environments DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Institute for Occupational Safety and Health Cover photo by Thinkstock© Criteria for a Recommended Standard Occupational Exposure to Heat and Hot Environments Revised Criteria 2016 Brenda Jacklitsch, MS; W. Jon Williams, PhD; Kristin Musolin, DO, MS; Aitor Coca, PhD; Jung-Hyun Kim, PhD; Nina Turner, PhD DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Institute for Occupational Safety and Health This document is in the public domain and may be freely copied or reprinted. Disclaimer Mention of any company or product does not constitute endorsement by the National Institute for Occupational Safety and Health (NIOSH). In addition, citations of websites external to NIOSH do not constitute NIOSH endorsement of the sponsoring organizations or their programs or products. Furthermore, NIOSH is not responsible for the content of these websites. Ordering Information This document is in the public domain and may be freely copied or reprinted. To receive NIOSH documents or other information about occupational safety and health topics, contact NIOSH at Telephone: 1-800-CDC-INFO (1-800-232-4636) TTY: 1-888-232-6348 E-mail: [email protected] or visit the NIOSH website at www.cdc.gov/niosh. For a monthly update on news at NIOSH, subscribe to NIOSH eNews by visiting www.cdc.gov/ niosh/eNews. Suggested Citation NIOSH [2016]. NIOSH criteria for a recommended standard: occupational exposure to heat and hot environments. By Jacklitsch B, Williams WJ, Musolin K, Coca A, Kim J-H, Turner N. -

12. Shortness of Breath
12. Shortness of breath Shortness of breath (dyspnea) is described as an intense tightening in the chest, air hunger, difficulty breathing, breathlessness or a feeling of suffocation. It is a subjective symptom of many diseases. The causes of shortness of breath can be various, but most often pulmonary or cardiac. The main causes of pulmonary or cardiac dyspnea include heart congestion, pulmonary embolism, emphysema, atelectasis, pneumothorax and interstitial lung processes. The basic imaging method in the diagnosis of dyspnea that follows clinical examination is a chest X- ray. It is used to evaluate heart congestion, fluid effusions, atelectasis, pneumothorax, and a rough assessment of interstitial lung processes. Only if there is a clinical suspicion of pulmonary embolization, the CT angiography (with intravenous contrast), perfusion lung scintigraphy or echocardiography are imaging methods of choice. If interstitial lung disease or pulmonary emphysema are suspected or there is uncertain finding on chest X-ray, high-resolution CT (HRCT) of the chest is recommended. HRCT allows detailed assessment of pulmonary parenchyma. Non-contrast CT is usually sufficient to assess interstitial changes. Heart congestion Heart congestion is the result of insufficient cardiac output due to heart failure or fluid overload. The most common cause of heart congestion is left heart failure with increased pressure in pulmonary veins and capillaries. On chest X- ray can be distinguished 3 degrees of heart congestion. Note: Right-sided heart failure most often occurs in long-term left-sided heart failure or lung disease, which leads to high pulmonary artery resistance (various etiologies of pulmonary hypertension, e.g. advanced COPD). -

Interstitial Lung Disease
Interstitial Lung Disease Camille Washowich, MSN, ACNP, CCRN Center for Advanced Lung Disease Stanford University Medical Center Lung Physiology ILD Classification Interstitial Lung Disease Connective Tissue Diseases Primary (unclassified) Idiopathic Fibrotic Disorders Drug and Treatment Induced Connective Tissue Diseases Scleroderma Systemic Lupus Erythematous (SLE) Rheumatoid Arthritis Mixed Connective Tissue Disease Primary (unclassified) Sarcoidosis Stage I-IV Neurofibromatosis Tuberous Sclerosis AIDS ARDS Bone Marrow Transplantation Post infectious Occupational & Environmental Exposures: Inorganic & Organic Agriculture Workers and Animal Handlers Construction: wood/metal Auto repair Military Chemicals (plastic, paint, polyurethane) Organisms: fungus/molds/bacterium Idiopathic Fibrotic Disorders Pulmonary fibrosis Familial pulmonary fibrosis Autoimmune pulmonary fibrosis Respiratory bronchiolitis Nonspecific interstitial pneumonitis (NSIP) Drug Induced Antibiotics Anti-arrhythmics Anti-inflammatory Anti-convulsant Radiation/Chemotherapy Oxygen toxicity Narcotics ILD Epidemiology in the US 100K admissions/year Occupation DILD Sarcoidosis 11% 15% pulmonologist patients 5% DAH 8% 4% CTD Incidence: 5/100K 9% Men (31%) versus Women (26%) Other 11% IPF 45% of all ILD patients Pulmonary Fibrosis 52% Age/Gender/Race Specifications to Assist in Diagnosis 20-40yrs: Inherited Interstitial Lung diseases Familial idiopathic pulmonary fibrosis Collagen vascular disease- associated ILD LAM Pulmonary Langerhans’ cell granulomatosis Sarcoidosis 50yrs: -

Heat Related Illnesses
Heat Related Illnesses Refresher Course for the Family Physician 4/3/20 Brooks J. Obr MD MME University of Iowa Hospitals and Clinics Department of Emergency Medicine What we’ll cover… • Basics of heat related illnesses • Risk factors/etiology • Heat cramps • Prickly heat • Heat edema • Heat syncope • Heat exhaustion • Heat stroke • Workups, treatments/cooling measures, dispositions Let’s start with the basics… • Wide range of progressively more severe illnesses • Increasingly overwhelming heat stress • Basic dehydration Thermoregulatory dysfunction/organ failure • Normally, body temperature is maintained by balancing heat production with heat loss/dissipation Etiology • Pre-existing conditions hindering the body’s ability to dissipate heat predispose for heat-related illness • Age extremes • Dehydration (gastroenteritis, inadequate fluid intake, etc.) • Cardiovascular disease (CHF, CAD, etc.) • Obesity • Diabetes mellitus, hyperthyroidism, pheochromocytoma • Febrile illness • Skin diseases that hinder sweating (psoriasis, eczema, cystic fibrosis, scleroderma, etc.) Etiology • Pharmacologic contributors • Sympathomimetics • LSD, PCP, Cocaine • MAO inhibitors, antipsychotics, anxiolytics • Anticholinergics • Antihistamines • Beta-blockers • Diuretics • Laxatives • Drug/ETOH withdrawal Etiology • Environmental factors • Excessive heat/humidity • Prolonged exertion • Lack of mobility • Lack of air conditioning • Lack of acclimatization • Occlusive, nonporous clothing Pediatrics • A special note on pediatric patients: Children are at increased -

Hyperthermia & Heat Stroke: Heat-Related Conditions
Hyperthermia & Heat Stroke: Heat-Related Conditions Joseph Rampulla, MS, APRN,BC eat-related conditions occur when excess heat taxes or overwhelms the body’s thermoregulatory mechanisms. Heat illness is preventable and occurs more Hcommonly than most clinicians realize. Heat illness most seriously affects the poor, urban-dwellers, young children, those with chronic physical and mental illnesses, substance abusers, the elderly, and people who engage in excessive physical The exposure to activity under harsh conditions. While considerable overlap occurs, the important the heat and the concrete during the syndromes are: heat stroke, heat exhaustion, and heat cramps. Heat stroke is a life- hot summer months places many rough threatening emergency and occurs when the loss of thermoregulatory control results sleepers at great risk in hyperpyrexia (very high fever) and severe damage to many internal organs. for heat stroke and hyperthermia. Photo by Epidemiology Sharon Morrison RN Heat illness is generally underreported, and the deaths than all other natural disasters combined in true incidence is unknown. Death rates from other the USA. The elderly, the very poor, and socially causes (e.g. cardiovascular, respiratory) increase isolated individuals are disproportionately affected during heat waves but are generally not reflected in by heat waves. For example, death records during the morbidity and mortality statistics related to heat heat waves invariably include many elders who died illness. Nonetheless, heat waves account for more alone in hot apartments. Age 65 years, chronic The Health Care of Homeless Persons - Part II - Hyperthermia and Heat Stroke 199 illness, and residence in a poor neighborhood are greater than 65. -

Chest Radiology: a Resident's Manual
Chest Radiology: A Resident's Manual Bearbeitet von Johannes Kirchner 1. Auflage 2011. Buch. 300 S. Hardcover ISBN 978 3 13 153871 0 Format (B x L): 23 x 31 cm Weitere Fachgebiete > Medizin > Sonstige Medizinische Fachgebiete > Radiologie, Bildgebende Verfahren Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. 1 Heart Failure Acute left heart failure is most commonly caused by a hyperten- " Compare pulmonary vessels that are equidistant to a central sive crisis. Radiographic signs on the plain chest radiograph ob- point in the respective hilum. tained with the patient standing include: " Compare the diameter of a random easily identifiable superior " Redistribution of pulmonary perfusion lobe artery (often the anterior segmental artery is most easily " Presence of interstitial patterns (Kerley lines, peribronchial identifiable) with the diameter of the corresponding ipsilateral cuffing) bronchus (Fig. 1.62). " Alveolar densities with indistinct vascular structures (ad- vanced stage) As the pulmonary artery and corresponding ipsilateral bronchus " Pleural effusions are normally of precisely equal diameter, a larger arterial diameter is indicative of redistribution of perfusion (Fig. 1.63). The diagnos- All of these signs are essentially attributable to increased fluid tic criteria of caudal-to-cranial redistribution cannot be evaluated content in the abnormally heavy “wet” lung. The fluid accumula- on radiographs obtained in the supine patient. -

Pleural Effusion, Hypovascularity in Lung Zone (Westermark’S Sign) & Pyramid Shape Infiltrate with Peak Directed to Hilus (Hampton’S Hump)
Author(s): Michele M. Nypaver, MD, 2011 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/ We have reviewed this material in accordance with U.S. Copyright Law and have tried to maximize your ability to use, share, and adapt it. Copyright holders of content included in this material should contact [email protected] with any questions, corrections, or clarification regarding the use of content. For more information about how to cite these materials visit http://open.umich.edu/privacy-and-terms-use. Any medical information in this material is intended to inform and educate and is not a tool for self-diagnosis or a replacement for medical evaluation, advice, diagnosis or treatment by a healthcare professional. Please speak to your physician if you have questions about your medical condition. Viewer discretion is advised: Some medical content is graphic and may not be suitable for all viewers. Citation Key for more information see: http://open.umich.edu/wiki/CitationPolicy Use + Share + Adapt { Content the copyright holder, author, or law permits you to use, share and adapt. } Public Domain – Government: Works that are produced by the U.S. Government. (17 USC § 105) Public Domain – Expired: Works that are no longer protected due to an expired copyright term. Public Domain – Self Dedicated: Works that a copyright holder has dedicated to the public domain. Creative Commons – Zero Waiver Creative Commons – Attribution License Creative Commons – Attribution Share Alike License Creative Commons – Attribution Noncommercial License Creative Commons – Attribution Noncommercial Share Alike License GNU – Free Documentation License Make Your Own Assessment { Content Open.Michigan believes can be used, shared, and adapted because it is ineligible for copyright. -

Exertional Heat Illnesses Helen M
Journal of Athletic Training 2002;37(3):329±343 q by the National Athletic Trainers' Association, Inc www.journalofathletictraining.org National Athletic Trainers' Association Position Statement: Exertional Heat Illnesses Helen M. Binkley*; Joseph Beckett²; Douglas J. Casa³; Douglas M. Kleiner§; Paul E. Plummer\ *Mesa State College, Grand Junction, CO; ²University of Charleston, Charleston, WV; ³University of Connecticut, Storrs, CT; §University of Florida, Jacksonville, FL; \Indiana State University, Terre Haute, IN Helen M. Binkley, PhD, ATC, CSCS*D, NSCA-CPT (Chair), contributed to conception and design; acquisition of the data; and drafting, critical revision, and ®nal approval of the article. Joseph Beckett, EdD, ATC, contributed to acquisition of the data and drafting, critical revision, and ®nal approval of the article. Douglas J. Casa, PhD, ATC, FACSM, contributed to conception and design; acquisition of the data; and drafting, critical revision, and ®nal approval of the article. Douglas M. Kleiner, PhD, ATC, FACSM, and Paul E. Plummer, MA, ATC, contributed to acquisition of the data and drafting, critical revision, and ®nal approval of the article. Address correspondence to National Athletic Trainers' Association, Communications Department, 2952 Stemmons Freeway, Dallas, TX 75247. Objective: To present recommendations for the prevention, Recommendations: Certi®ed athletic trainers and other al- recognition, and treatment of exertional heat illnesses and to lied health providers should use these recommendations to es- describe the relevant physiology of thermoregulation. tablish on-site emergency plans for their venues and athletes. Background: Certi®ed athletic trainers evaluate and treat The primary goal of athlete safety is addressed through the heat-related injuries during athletic activity in ``safe'' and high- prevention and recognition of heat-related illnesses and a well- risk environments.