Diagnosing Pulmonary Embolism M Riedel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pleural Effusion and Ventilation/Perfusion Scan Interpretation for Acute Pulmonary Embolus
ventricular function by radionuclide angiography during exercise in normal subjects 33. Verani MS. Myocardial perfusion imaging versus two-dimensional echocardiography: and patients with chronic coronary heart disease. J Am Coll Cardiol 1983;1:1518- comparative value in the diagnosis of coronary artery disease. J NucÃCardiol 1529. 1994;1:399-414. 29. Adam WE. Tarkowska A, Bitter F, Stauch M, Geffers H. Equilibrium (gated) 34. Foster T, McNeill AJ, Salustri A, et al. Simultaneous dobutamine stress echocardiog radionuclide ventriculography. Cardiovasc Radial 1979;2:161-173. raphy and technetium-99m SPECT in patients with suspected coronary artery disease. 30. Hurwitz RA, TrêvesS, Kuroc A. Right ventricular and left ventricular ejection fraction J Am Coll Cardiol I993;21:1591-I596. in pediatrie patients with normal hearts: first pass radionuclide angiography. Am 35. Marwick TH, D'Hondt AM, Mairesse GH, Baudhuin T, Wijins W, Detry JM, Meiin Heart J 1984;107:726-732. 31. Freeman ML, Palac R, Mason J, et al. A comparison of dobutamine infusion and JA. Comparative ability of dobutamine and exercise stress in inducing myocardial ischemia in active patients. Br Heart J 1994:72:31-38. supine bicycle exercise for radionuclide cardiac stress testing. Clin NucÃMed 1984:9:251-255. 36. Senior R, Sridhara BS, Anagnostou E, Handler C, Raftery EB, Lahiri A. Synergistic 32. Cohen JL, Greene TO, Ottenweller J, Binebaum SZ, Wilchfort SD, Kim CS. value of simultaneous stress dobutamine sestamibi single-photon-emission computer Dobutamine digital echocardiography for detecting coronary artery disease. Am J ized tomography and echocardiography in the detection of coronary artery disease. -

An Interesting Case of Undiagnosed Pleural Effusion Case Report
Amit Panjwani, Thuraya Zaid [email protected] Pulmonary Medicine, Salmaniya Medical Complex, Manama, Bahrain. An interesting case of undiagnosed pleural effusion Case report Pleural effusions are commonly encountered in the Investigations revealed a haemoglobin level Cite as: Panjwani A, Zaid T. clinical practise of both respiratory and nonrespiratory of 16.4 g⋅dL−1, and total leukocyte count of An interesting case of specialists. An estimated 1–1.5 million new cases in 8870 cells⋅mm−3 with a differential count of 62% undiagnosed pleural effusion. the USA and 200 000–250 000 new cases of pleural neutrophils, 28% lymphocytes, 7% monocytes, 2% Breathe 2017; 13: e46–e52. effusions are reported from the UK each year [1]. eosinophils and 1% basophils. The platelet count Analysis of the relevant clinical history, physical was 160 000 cells⋅mm−3. Creatinine, electrolytes examination, chest radiography and diagnostic and liver function tests were normal. The ECG was thoracentesis is useful in identifying the cause of unremarkable and cardiac enzymes were within pleural effusion in majority of the cases [2]. In a few normal limits. Chest radiograph (figure 1) showed a cases, the aetiology may be unclear after the initial mild, right-sided pleural effusion, blunting of the left assessment. The list of diseases that may account for costophrenic angle, no shift of mediastinal position a persistent undiagnosed pleural effusion is long [3]. and no lung parenchymal opacities. We present an interesting case of undiagnosed pleural effusion that was encountered in our hospital. R Case presentation A 33-year-old male presented to our hospital with a history of sudden-onset, pleuritic, right-sided chest pain of 2 days’ duration. -

Diagnosis of Chronic Thromboembolic Pulmonary Hypertension After Acute Pulmonary Embolism
Early View Review Diagnosis of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism Fredrikus A. Klok, Francis Couturaud, Marion Delcroix, Marc Humbert Please cite this article as: Klok FA, Couturaud F, Delcroix M, et al. Diagnosis of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Eur Respir J 2020; in press (https://doi.org/10.1183/13993003.00189-2020). This manuscript has recently been accepted for publication in the European Respiratory Journal. It is published here in its accepted form prior to copyediting and typesetting by our production team. After these production processes are complete and the authors have approved the resulting proofs, the article will move to the latest issue of the ERJ online. Copyright ©ERS 2020 Diagnosis of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism Fredrikus A. Klok, Francis Couturaud F2, Marion Delcroix M3, Marc Humbert4-6 1 Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, The Netherlands 2 Département de Médecine Interne et Pneumologie, Centre Hospitalo-Universitaire de Brest, Univ Brest, EA 3878, CIC INSERM1412, Brest, France 3 Department of Respiratory Diseases, University Hospitals and Respiratory Division, Department of Chronic Diseases, Metabolism & Aging, KU Leuven – University of Leuven, Leuven, Belgium 4 Université Paris-Saclay, Faculté de Médecine, Le Kremlin-Bicêtre, France 5 Service de Pneumologie et Soins Intensifs Respiratoires, Hôpital Bicêtre, AP-HP, Le Kremlin-Bicêtre, France 6 INSERM UMR S 999, Hôpital Marie Lannelongue, Le Plessis Robinson, France Corresponding author: Frederikus A. Klok, MD, FESC; Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, the Netherlands; Albinusdreef 2, 2300RC, Leiden, the Netherlands; Phone: +31- 715269111; E-mail: [email protected] Abstract Chronic thromboembolic pulmonary hypertension (CTEPH) is the most severe long-term complication of acute pulmonary embolism (PE). -
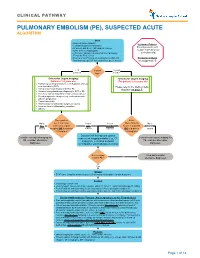
Pulmonary Embolism (Pe), Suspected Acute Algorithm
CLINICAL PATHWAY PULMONARY EMBOLISM (PE), SUSPECTED ACUTE ALGORITHM Start · Room air pulse oximetry Inclusion Criteria: · Consider supplemental oxygen · IV access and STAT CBC and DIC screen Patient presents with · Urine β-HCG, if appropriate suspected Pulmonary · CXR (PA + lateral), EKG and call the Cardiology Embolism (PE) Fellow for Echo review · Review criteria* for urgent imaging for possible PE Exclusion Criteria based on age-specific risk factors (see green boxes) No suspected PE < 18 Patient 18 years years old age? or older *Criteria for Urgent Imaging: *Criteria for Urgent Imaging: Patients < 18 years old For patients ≥ 18 years old · Painful leg swelling or known recent diagnosis of deep vein thrombosis (DVT) Please refer to the Wells Criteria · Family or personal history of DVT or PE Algorithm on page 7. · Known clotting disorder predisposing to DVT or PE · Recent or current indwelling central venous catheter · Elevated systemic estrogen (e.g., oral contraceptive pill use, pregnancy) · Recent immobility · Recent major or orthopedic surgery or trauma · Acute or chronic inflammatory condition · Obesity Does patient Is the No to meet 1 or more Yes to Yes to Wells Criteria No to ALL criteria for urgent ANY EITHER Score > 4 points BOTH criteria imaging OR is d-dimer criteria criterion OR is d-dimer criteria > 0.5ug/mL? > 0.5ug/mL? Discuss CXR findings and optimal Consider non-urgent imaging for subsequent imaging modality (e.g., CT Consider non-urgent imaging for PE, consider alternative angiogram, ventilation perfusion PE, consider -

Acute Pulmonary Embolism in Patients with and Without COVID-19
Journal of Clinical Medicine Article Acute Pulmonary Embolism in Patients with and without COVID-19 Antonin Trimaille 1,2 , Anaïs Curtiaud 1, Kensuke Matsushita 1,2 , Benjamin Marchandot 1 , Jean-Jacques Von Hunolstein 1 , Chisato Sato 1,2, Ian Leonard-Lorant 3, Laurent Sattler 4 , Lelia Grunebaum 4, Mickaël Ohana 3 , Patrick Ohlmann 1 , Laurence Jesel 1,2 and Olivier Morel 1,2,* 1 Division of Cardiovascular Medicine, Nouvel Hôpital Civil, Strasbourg University Hospital, 67000 Strasbourg, France; [email protected] (A.T.); [email protected] (A.C.); [email protected] (K.M.); [email protected] (B.M.); [email protected] (J.-J.V.H.); [email protected] (C.S.); [email protected] (P.O.); [email protected] (L.J.) 2 INSERM (French National Institute of Health and Medical Research), UMR 1260, Regenerative Nanomedicine, FMTS, 67000 Strasbourg, France 3 Radiology Department, Nouvel Hôpital Civil, Strasbourg University Hospital, 67000 Strasbourg, France; [email protected] (I.L.-L.); [email protected] (M.O.) 4 Haematology and Haemostasis Laboratory, Centre for Thrombosis and Haemostasis, Nouvel Hôpital Civil, Strasbourg University Hospital, 67000 Strasbourg, France; [email protected] (L.S.); [email protected] (L.G.) * Correspondence: [email protected] Abstract: Introduction. Acute pulmonary embolism (APE) is a frequent condition in patients with Citation: Trimaille, A.; Curtiaud, A.; COVID-19 and is associated with worse outcomes. Previous studies suggested an immunothrombosis Matsushita, K.; Marchandot, B.; instead of a thrombus embolism, but the precise mechanisms remain unknown. -

COVID-19 Associated Pulmonary Embolism in Pediatric Patients
Prepublication Release A N O F F I C I A L J O U R N A L O F T H E A M E R I C A N A C A D E M Y O F P E D I A T R I C S COVID-19 Associated Pulmonary Embolism in Pediatric Patients Melissa Chima, Duane Williams, Neal J. Thomas, Conrad Krawiec DOI: 10.1542/hpeds.2021-005866 Journal: Hospital Pediatrics Article Type: Original Article Citation: Chima M, et al. COVID-19 Associated Pulmonary Embolism in Pediatric Patients. Hosp Pediatr. 2021; doi: 10.1542/hpeds.2021-005866 This is a prepublication version of an article that has undergone peer review and been accepted for publication but is not the final version of record. This paper may be cited using the DOI and date of access. This paper may contain information that has errors in facts, figures, and statements, and will be corrected in the final published version. The journal is providing an early version of this article to expedite access to this information. The American Academy of Pediatrics, the editors, and authors are not responsible for inaccurate information and data described in this version. Downloaded©202 from1 www.aappublications.org/news American Academy byof guest Pediatrics on October 1, 2021 Prepublication Release COVID-19 Associated Pulmonary Embolism in Pediatric Patients Melissa Chima, BS1, Duane Williams, MD2, Neal J. Thomas, MD2,3, Conrad Krawiec, MD2 Authors’ Affiliations and Addresses: 1Penn State College of Medicine, 500 University Drive, P.O. Box 850, Hershey, PA, USA 17033-0850, Tel: (717)-531-5337, Fax: (717)-531-8985. -

Chapter 17 Dyspnea Sabina Braithwaite and Debra Perina
Chapter 17 Dyspnea Sabina Braithwaite and Debra Perina ■ PERSPECTIVE Pathophysiology Dyspnea is the term applied to the sensation of breathlessness The actual mechanisms responsible for dyspnea are unknown. and the patient’s reaction to that sensation. It is an uncomfort- Normal breathing is controlled both centrally by the respira- able awareness of breathing difficulties that in the extreme tory control center in the medulla oblongata, as well as periph- manifests as “air hunger.” Dyspnea is often ill defined by erally by chemoreceptors located near the carotid bodies, and patients, who may describe the feeling as shortness of breath, mechanoreceptors in the diaphragm and skeletal muscles.3 chest tightness, or difficulty breathing. Dyspnea results Any imbalance between these sites is perceived as dyspnea. from a variety of conditions, ranging from nonurgent to life- This imbalance generally results from ventilatory demand threatening. Neither the clinical severity nor the patient’s per- being greater than capacity.4 ception correlates well with the seriousness of underlying The perception and sensation of dyspnea are believed to pathology and may be affected by emotions, behavioral and occur by one or more of the following mechanisms: increased cultural influences, and external stimuli.1,2 work of breathing, such as the increased lung resistance or The following terms may be used in the assessment of the decreased compliance that occurs with asthma or chronic dyspneic patient: obstructive pulmonary disease (COPD), or increased respira- tory drive, such as results from severe hypoxemia, acidosis, or Tachypnea: A respiratory rate greater than normal. Normal rates centrally acting stimuli (toxins, central nervous system events). -

Pulmonary Embolism Or Pneumocystis Jiroveci Pneumonia?
breathe case presentations.qxd 26/07/2006 12:03 Page 5 CASE PRESENTATION Pulmonary embolism or Pneumocystis jiroveci pneumonia? Case report Table 1 Vital signs and F. Braiteh1,2 A 33-year-old male presented to the emergency laboratory test results at I. Nash3 department with a 5-day history of exertional dys- presentation pnoea, dry cough, lethargy and an ongoing fever of 38.9°C. He had been previously diagnosed Investigation Result Normal 1Medical Oncology, Division of with left-frontal oligodendroglioma during a range Cancer Medicine, The University work-up following a new-onset seizure 4 months Vital signs of Texas M.D. Anderson Cancer Temperature °C 36.6 earlier. After successful tumour resection and Center, 2University of Texas Respiratory rate cycles·min-1 22 adjuvant radiotherapy, the patient totally recov- Graduate School of Biomedical Heart rate beats·min-1 88 ered without any residual paresis. He was main- Sciences, Houston, TX, and Blood pressure mmHg 126/64 3Dept of Pathology, Hospital of tained on valproic acid and dexamethasone at a O2 saturation % 91 Saint Raphael, Yale School of -1 dose that was tapered down to 2 mg·day . Haematological counts and coagulation Medicine, New Haven, CT, USA. The physical examination was unremarkable. White cells ×109·L -1 6.8 4.0–10.0 The patient was haemodynamically stable but Platelets ×109·L -1 123 150–350 hypoxaemic and anaemic (table 1). Chest radio- Haemoglobin g·dL-1 9.3 12.0–16.0 Correspondence: graphy and computed tomography (CT) were Prothrombine time s 12.8 <13.0 F. -

Pulmonary Embolism (Pe): Diagnosis
PULMONARY EMBOLISM (PE): DIAGNOSIS OBJECTIVE: To provide a diagnostic approach to patients with suspected acute pulmonary embolism (PE). BACKGROUND: Venous thromboembolism (VTE), which comprises deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common disease, affecting approximately 1-2 in 1,000 adults per year. The diagnosis of PE has increased significantly since the advent of computed tomography pulmonary angiography (CTPA) with its widespread availability and enhanced sensitivity. The majority of PE originates in the proximal deep veins of the leg, despite the observation that only 25-50% of patients with PE have clinically evident DVT at the time of PE diagnosis. While active malignancy, surgery (especially orthopedic), hospitalization, air travel >8 hours, and hormone use/pregnancy are common transient provoking factors, approximately 50% of first-time PEs appear to be unprovoked. Symptoms of PE may include sudden onset dyspnea, pleuritic chest pain, and syncope. Signs of PE may include tachypnea, tachycardia, hypoxemia, hypotension, and features of right ventricular dysfunction (distended jugular veins). There may be accompanying signs and symptoms of DVT. The ECG may show right ventricular strain (S1Q3T3, right bundle branch block and T-inversion in leads V1- V4). Up to 10% of symptomatic PEs are fatal within the first hour of symptom onset. Independent predictors of mortality within the first few days after diagnosis of PE include hypotension (systolic blood pressure [SBP] <90 mmHg), clinical right heart failure, right ventricular dilatation on CTPA/echocardiography, positive troponin, and elevated brain natriuretic peptide (BNP). Early diagnosis and treatment of PE reduces morbidity and mortality. DIAGNOSIS OF PE: The constellation of symptoms and signs may be suggestive of PE but do not alone have the sensitivity or specificity to rule in or rule out the diagnosis. -

Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE)
How can it be prevented? You can take steps to prevent deep vein thrombosis (DVT) and pulmonary embolism (PE). If you're at risk for these conditions: • See your doctor for regular checkups. • Take all medicines as your doctor prescribes. • Get out of bed and move around as soon as possible after surgery or illness (as your doctor recommends). Moving around lowers your chance of developing a blood clot. References: • Exercise your lower leg muscles during Deep Vein Thrombosis: MedlinePlus. (n.d.). long trips. Walking helps prevent blood Retrieved October 18, 2016, from clots from forming. https://medlineplus.gov/deepveinthrombos is.html If you've had DVT or PE before, you can help prevent future blood clots. Follow the steps What Are the Signs and Symptoms of Deep above and: Vein Thrombosis? - NHLBI, NIH. (n.d.). Retrieved October 18, 2016, from • Take all medicines that your doctor http://www.nhlbi.nih.gov/health/health- prescribes to prevent or treat blood clots topics/topics/dvt/signs • Follow up with your doctor for tests and treatment Who Is at Risk for Deep Vein Thrombosis? - • Use compression stockings as your DEEP NHLBI, NIH. (n.d.). Retrieved October 18, doctor directs to prevent leg swelling 2016, from http://www.nhlbi.nih.gov/health/health- VEIN topics/topics/dvt/atrisk THROMBOSIS How Can Deep Vein Thrombosis Be Prevented? - NHLBI, NIH. (n.d.). Retrieved October 18, 2016, from (DVT) http://www.nhlbi.nih.gov/health/health- topics/topics/dvt/prevention How Is Deep Vein Thrombosis Treated? - NHLBI, NIH. (n.d.). Retrieved October 18, 2016, from http://www.nhlbi.nih.gov/health/health- topics/topics/dvt/treatment Trinity Surgery Center What is deep vein Who is at risk? What are the thrombosis (DVT)? The risk factors for deep vein thrombosis symptoms? (DVT) include: Only about half of the people who have DVT A blood clot that forms in a vein deep in the • A history of DVT. -

Mayo Clinic Medical Manual This Page Intentionally Left Blank Mayo Clinic Medical Manual
Mayo Clinic Medical Manual This page intentionally left blank Mayo Clinic Medical Manual Editors Guilherme H. M. Oliveira, M.D. Gillian C. Nesbitt, M.D. Joseph G. Murphy, M.D. MAYO CLINIC SCIENTIFIC PRESS TAYLOR & FRANCIS GROUP ISBN 0849390877 The triple-shield Mayo logo and the words MAYO, MAYO CLINIC, and MAYO CLINIC SCIENTIFIC PRESS are marks of Mayo Foundation for Medical Education and Research. ©2006 by Mayo Foundation for Medical Education and Research. All rights reserved. This book is protected by copyright. No part of it may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means—electronic, mechanical, photocopying, record- ing, or otherwise—without the prior written consent of the copyright holder, except for brief quotations embodied in critical articles and reviews. Inquiries should be addressed to Scientific Publications, Plummer 10, Mayo Clinic, 200 First Street SW, Rochester, MN 55905. For order inquiries, contact Taylor & Francis Group, 6000 Broken Sound Parkway NW, Suite #300, Boca Raton, FL 33487. www.taylorandfrancis.com Catalog record is available from the Library of Congress. Care has been taken to confirm the accuracy of the information presented and to describe generally accepted practices. However, the authors, editors, and publisher are not responsible for errors or omissions or for any consequences from application of the information in this book and make no warranty, express or implied, with respect to the contents of the publication. This book should not be relied on apart from the advice of a qualified health care provider. The authors, editors, and publisher have exerted efforts to ensure that drug selection and dosage set forth in this text are in accordance with current recommendations and practice at the time of publication. -

Pleural Effusion, Hypovascularity in Lung Zone (Westermark’S Sign) & Pyramid Shape Infiltrate with Peak Directed to Hilus (Hampton’S Hump)
Author(s): Michele M. Nypaver, MD, 2011 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/ We have reviewed this material in accordance with U.S. Copyright Law and have tried to maximize your ability to use, share, and adapt it. Copyright holders of content included in this material should contact [email protected] with any questions, corrections, or clarification regarding the use of content. For more information about how to cite these materials visit http://open.umich.edu/privacy-and-terms-use. Any medical information in this material is intended to inform and educate and is not a tool for self-diagnosis or a replacement for medical evaluation, advice, diagnosis or treatment by a healthcare professional. Please speak to your physician if you have questions about your medical condition. Viewer discretion is advised: Some medical content is graphic and may not be suitable for all viewers. Citation Key for more information see: http://open.umich.edu/wiki/CitationPolicy Use + Share + Adapt { Content the copyright holder, author, or law permits you to use, share and adapt. } Public Domain – Government: Works that are produced by the U.S. Government. (17 USC § 105) Public Domain – Expired: Works that are no longer protected due to an expired copyright term. Public Domain – Self Dedicated: Works that a copyright holder has dedicated to the public domain. Creative Commons – Zero Waiver Creative Commons – Attribution License Creative Commons – Attribution Share Alike License Creative Commons – Attribution Noncommercial License Creative Commons – Attribution Noncommercial Share Alike License GNU – Free Documentation License Make Your Own Assessment { Content Open.Michigan believes can be used, shared, and adapted because it is ineligible for copyright.