Clinical and Imaging Profiles of Pulmonary Embolism: a Single
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diagnosing Pulmonary Embolism M Riedel
309 Postgrad Med J: first published as 10.1136/pgmj.2003.007955 on 10 June 2004. Downloaded from REVIEW Diagnosing pulmonary embolism M Riedel ............................................................................................................................... Postgrad Med J 2004;80:309–319. doi: 10.1136/pgmj.2003.007955 Objective testing for pulmonary embolism is necessary, embolism have a low long term risk of subse- quent VTE.2 5–7 because clinical assessment alone is unreliable and the consequences of misdiagnosis are serious. No single test RISK FACTORS AND RISK has ideal properties (100% sensitivity and specificity, no STRATIFICATION risk, low cost). Pulmonary angiography is regarded as the The factors predisposing to VTE broadly fit Virchow’s triad of venous stasis, injury to the final arbiter but is ill suited for diagnosing a disease vein wall, and enhanced coagulability of the present in only a third of patients in whom it is suspected. blood (box 1). The identification of risk factors Some tests are good for confirmation and some for aids clinical diagnosis of VTE and guides decisions about repeat testing in borderline exclusion of embolism; others are able to do both but are cases. Primary ‘‘thrombophilic’’ abnormalities often non-diagnostic. For optimal efficiency, choice of the need to interact with acquired risk factors before initial test should be guided by clinical assessment of the thrombosis occurs; they are usually discovered after the thromboembolic event. Therefore, the likelihood of embolism and by patient characteristics that risk of VTE is best assessed by recognising the may influence test accuracy. Standardised clinical presence of known ‘‘clinical’’ risk factors. estimates can be used to give a pre-test probability to However, investigations for thrombophilic dis- orders at follow up should be considered in those assess, after appropriate objective testing, the post-test without another apparent explanation. -
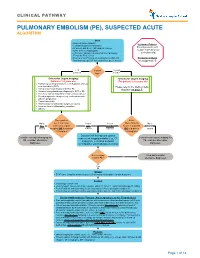
Pulmonary Embolism (Pe), Suspected Acute Algorithm
CLINICAL PATHWAY PULMONARY EMBOLISM (PE), SUSPECTED ACUTE ALGORITHM Start · Room air pulse oximetry Inclusion Criteria: · Consider supplemental oxygen · IV access and STAT CBC and DIC screen Patient presents with · Urine β-HCG, if appropriate suspected Pulmonary · CXR (PA + lateral), EKG and call the Cardiology Embolism (PE) Fellow for Echo review · Review criteria* for urgent imaging for possible PE Exclusion Criteria based on age-specific risk factors (see green boxes) No suspected PE < 18 Patient 18 years years old age? or older *Criteria for Urgent Imaging: *Criteria for Urgent Imaging: Patients < 18 years old For patients ≥ 18 years old · Painful leg swelling or known recent diagnosis of deep vein thrombosis (DVT) Please refer to the Wells Criteria · Family or personal history of DVT or PE Algorithm on page 7. · Known clotting disorder predisposing to DVT or PE · Recent or current indwelling central venous catheter · Elevated systemic estrogen (e.g., oral contraceptive pill use, pregnancy) · Recent immobility · Recent major or orthopedic surgery or trauma · Acute or chronic inflammatory condition · Obesity Does patient Is the No to meet 1 or more Yes to Yes to Wells Criteria No to ALL criteria for urgent ANY EITHER Score > 4 points BOTH criteria imaging OR is d-dimer criteria criterion OR is d-dimer criteria > 0.5ug/mL? > 0.5ug/mL? Discuss CXR findings and optimal Consider non-urgent imaging for subsequent imaging modality (e.g., CT Consider non-urgent imaging for PE, consider alternative angiogram, ventilation perfusion PE, consider -

Chapter 17 Dyspnea Sabina Braithwaite and Debra Perina
Chapter 17 Dyspnea Sabina Braithwaite and Debra Perina ■ PERSPECTIVE Pathophysiology Dyspnea is the term applied to the sensation of breathlessness The actual mechanisms responsible for dyspnea are unknown. and the patient’s reaction to that sensation. It is an uncomfort- Normal breathing is controlled both centrally by the respira- able awareness of breathing difficulties that in the extreme tory control center in the medulla oblongata, as well as periph- manifests as “air hunger.” Dyspnea is often ill defined by erally by chemoreceptors located near the carotid bodies, and patients, who may describe the feeling as shortness of breath, mechanoreceptors in the diaphragm and skeletal muscles.3 chest tightness, or difficulty breathing. Dyspnea results Any imbalance between these sites is perceived as dyspnea. from a variety of conditions, ranging from nonurgent to life- This imbalance generally results from ventilatory demand threatening. Neither the clinical severity nor the patient’s per- being greater than capacity.4 ception correlates well with the seriousness of underlying The perception and sensation of dyspnea are believed to pathology and may be affected by emotions, behavioral and occur by one or more of the following mechanisms: increased cultural influences, and external stimuli.1,2 work of breathing, such as the increased lung resistance or The following terms may be used in the assessment of the decreased compliance that occurs with asthma or chronic dyspneic patient: obstructive pulmonary disease (COPD), or increased respira- tory drive, such as results from severe hypoxemia, acidosis, or Tachypnea: A respiratory rate greater than normal. Normal rates centrally acting stimuli (toxins, central nervous system events). -

Mayo Clinic Medical Manual This Page Intentionally Left Blank Mayo Clinic Medical Manual
Mayo Clinic Medical Manual This page intentionally left blank Mayo Clinic Medical Manual Editors Guilherme H. M. Oliveira, M.D. Gillian C. Nesbitt, M.D. Joseph G. Murphy, M.D. MAYO CLINIC SCIENTIFIC PRESS TAYLOR & FRANCIS GROUP ISBN 0849390877 The triple-shield Mayo logo and the words MAYO, MAYO CLINIC, and MAYO CLINIC SCIENTIFIC PRESS are marks of Mayo Foundation for Medical Education and Research. ©2006 by Mayo Foundation for Medical Education and Research. All rights reserved. This book is protected by copyright. No part of it may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means—electronic, mechanical, photocopying, record- ing, or otherwise—without the prior written consent of the copyright holder, except for brief quotations embodied in critical articles and reviews. Inquiries should be addressed to Scientific Publications, Plummer 10, Mayo Clinic, 200 First Street SW, Rochester, MN 55905. For order inquiries, contact Taylor & Francis Group, 6000 Broken Sound Parkway NW, Suite #300, Boca Raton, FL 33487. www.taylorandfrancis.com Catalog record is available from the Library of Congress. Care has been taken to confirm the accuracy of the information presented and to describe generally accepted practices. However, the authors, editors, and publisher are not responsible for errors or omissions or for any consequences from application of the information in this book and make no warranty, express or implied, with respect to the contents of the publication. This book should not be relied on apart from the advice of a qualified health care provider. The authors, editors, and publisher have exerted efforts to ensure that drug selection and dosage set forth in this text are in accordance with current recommendations and practice at the time of publication. -

Pleural Effusion, Hypovascularity in Lung Zone (Westermark’S Sign) & Pyramid Shape Infiltrate with Peak Directed to Hilus (Hampton’S Hump)
Author(s): Michele M. Nypaver, MD, 2011 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/ We have reviewed this material in accordance with U.S. Copyright Law and have tried to maximize your ability to use, share, and adapt it. Copyright holders of content included in this material should contact [email protected] with any questions, corrections, or clarification regarding the use of content. For more information about how to cite these materials visit http://open.umich.edu/privacy-and-terms-use. Any medical information in this material is intended to inform and educate and is not a tool for self-diagnosis or a replacement for medical evaluation, advice, diagnosis or treatment by a healthcare professional. Please speak to your physician if you have questions about your medical condition. Viewer discretion is advised: Some medical content is graphic and may not be suitable for all viewers. Citation Key for more information see: http://open.umich.edu/wiki/CitationPolicy Use + Share + Adapt { Content the copyright holder, author, or law permits you to use, share and adapt. } Public Domain – Government: Works that are produced by the U.S. Government. (17 USC § 105) Public Domain – Expired: Works that are no longer protected due to an expired copyright term. Public Domain – Self Dedicated: Works that a copyright holder has dedicated to the public domain. Creative Commons – Zero Waiver Creative Commons – Attribution License Creative Commons – Attribution Share Alike License Creative Commons – Attribution Noncommercial License Creative Commons – Attribution Noncommercial Share Alike License GNU – Free Documentation License Make Your Own Assessment { Content Open.Michigan believes can be used, shared, and adapted because it is ineligible for copyright. -

Signs in Chest Imaging
Diagn Interv Radiol 2011; 17:18–29 CHEST IMAGING © Turkish Society of Radiology 2011 PICTORIAL ESSAY Signs in chest imaging Oktay Algın, Gökhan Gökalp, Uğur Topal ABSTRACT adiological practice includes classification of illnesses with similar A radiological sign can sometimes resemble a particular object characteristics through recognizable signs. Knowledge of and abil- or pattern and is often highly suggestive of a group of similar pathologies. Awareness of such similarities can shorten the dif- R ity to recognize these signs can aid the physician in shortening ferential diagnosis list. Many such signs have been described the differential diagnosis list and deciding on the ultimate diagnosis for for X-ray and computed tomography (CT) images. In this ar- ticle, we present the most frequently encountered plain film a patient. In this report, 23 important and frequently seen radiological and CT signs in chest imaging. These signs include for plain signs are presented and described using chest X-rays, computed tomog- films the air bronchogram sign, silhouette sign, deep sulcus raphy (CT) images, illustrations and photographs. sign, Continuous diaphragm sign, air crescent (“meniscus”) sign, Golden S sign, cervicothoracic sign, Luftsichel sign, scim- itar sign, doughnut sign, Hampton hump sign, Westermark Plain films sign, and juxtaphrenic peak sign, and for CT the gloved finger Air bronchogram sign sign, CT halo sign, signet ring sign, comet tail sign, CT an- giogram sign, crazy paving pattern, tree-in-bud sign, feeding Bronchi, which are not normally seen, become visible as a result of vessel sign, split pleura sign, and reversed halo sign. opacification of the lung parenchyma. -

Eponyms in Radiologic Signs
Eponyms in radiologic signs Poster No.: C-0133 Congress: ECR 2014 Type: Educational Exhibit Authors: D. Andrade, L. Andrade, M. Magalhaes, L. Curvo-Semedo, F. Caseiro Alves; Coimbra/PT Keywords: Diagnostic procedure, Fluoroscopy, CT, Conventional radiography, Thorax, Musculoskeletal system, Gastrointestinal tract, Education and training DOI: 10.1594/ecr2014/C-0133 Any information contained in this pdf file is automatically generated from digital material submitted to EPOS by third parties in the form of scientific presentations. References to any names, marks, products, or services of third parties or hypertext links to third- party sites or information are provided solely as a convenience to you and do not in any way constitute or imply ECR's endorsement, sponsorship or recommendation of the third party, information, product or service. ECR is not responsible for the content of these pages and does not make any representations regarding the content or accuracy of material in this file. As per copyright regulations, any unauthorised use of the material or parts thereof as well as commercial reproduction or multiple distribution by any traditional or electronically based reproduction/publication method ist strictly prohibited. You agree to defend, indemnify, and hold ECR harmless from and against any and all claims, damages, costs, and expenses, including attorneys' fees, arising from or related to your use of these pages. Please note: Links to movies, ppt slideshows and any other multimedia files are not available in the pdf version of presentations. www.myESR.org Page 1 of 43 Learning objectives 1. To recognize the most frequent and important radiologic signs that are eponyms. -

Case 1 Pt Is a 45 Yo Female Who Presents with a “Run Down Feeling” After Returning from a Trip to India
Case 1 Pt is a 45 yo female who presents with a “run down feeling” after returning from a trip to India. When talking with the patient you learn that she just hasn’t been able to perform the activities she normally could without having to rest. For example, a few weeks ago she was able to work on her house for approximately 12 hrs per day, but recently she is exhausted after a few hours of activity. The patient is not aware of an “exact” moment that the symptoms started, but noted it has not changed in intensity over the past two weeks. Activity worsens the feeling; rest improves her symptoms. The patient also complains of chest pain with deep breathing. Pt denies cough, wheezing, feelings of palpitations, hiccoughs, episodes of seizures or syncope. What things could be causing her problems? This is a patient who has decreased exercise capacity that came on fairly quickly. Things that could be causing this could involve decreased oxygenation of the blood or decreased pumping of well oxygenated blood. Additionally, build up of toxins, that should be cleared by the liver or kidney, can make people feel crummy. The list is very broad still, pneumonia, congestive heart failure (CHF), acute anemia, pericarditis, pulmonary embolism (PE), acute renal failure (ARF) viral illness, etc. What review of systems would you like to know? Any fever, change in weight, rash or jaundice, dizziness, cough, shortness of breath (SOB), wheezing, palpitations, new edema, abdominal pain, nausea/vomiting, GERD (gastroesophageal reflux disease) symptoms, BRBPR (bright red blood per rectum), melena, episodes of seizures or syncope, any signs of depression (SIG E CAPS- sleep, interests, guilt, energy, concentration/memory, affect/appetite, psychomotor changes, suicidal/sexuality/somatic symptoms) The patient says no to most of your questions, except his left lower leg seems larger than his right. -

Pulmonary Embolism : Dr SC Coka
PULMONARYPULMONARY EMBOLISMEMBOLISM --EDHEDH-- SEPTEMBERSEPTEMBER 20072007 DRDR SS CC COKACOKA CASECASE PRESENTATIONPRESENTATION Mrs.Mrs. N.N. MkhizeMkhize 5151 yryr oldold presentedpresented with:with: -- ShortnessShortness ofof breathbreath forfor oneone daysdays durationduration RiskRisk factors:factors: -- RaisedRaised BMIBMI -- StrongStrong familyfamily hxhx ofof MIMI-- fatherfather andand sistersister bothboth dieddied ofof MIMI inin theirtheir 5050’’ss NoNo otherother traditionaltraditional riskrisk factorsfactors OnOn EnquiryEnquiry:: -- GradeGrade 33 dyspnoeadyspnoea (NYHAC)(NYHAC) associatedassociated chestchest painpain underunder leftleft breastbreast radiatingradiating toto thethe backback describeddescribed asas stabbingstabbing inin naturenature NoNo nausea,nausea, vomittingvomitting nornor sweating.sweating. painpain waswas ofof suddensudden onsetonset atat restrest notnot relatedrelated toto mealsmeals nono identifiableidentifiable relievingrelieving nornor exacerbatingexacerbating factorsfactors --NoNo historyhistory ofof orthopnea/orthopnea/ PND/pedalPND/pedal oedemaoedema --NoNo historyhistory ofof coughcough nornor haempotysishaempotysis PMHPMH:: NoneNone ofof notenote PSH:PSH: varicosevaricose veinvein strippingstripping inin leftleft legleg inin 19931993 PreviousPrevious C/SC/S inin 19851985 SHSH:: SheShe isis ofof sobersober habitshabits FH:FH: FatherFather dieddied ofof MIMI atat ageage 56,56, sistersister alsoalso dieddied ofof MIMI atat ageage 5454 andand brotherbrother hashas unstableunstable anginaangina -

Chapter 105 Peripheral Nerve Disorders E
Chapter 105 Peripheral Nerve Disorders E. Bradshaw Bunney and E. John Gallagher ■ PERSPECTIVE oped countries, its annual incidence is just over 1 to 2 cases per 100,000 population.1 In contrast to the low incidence of Background acute peripheral neuropathies, several of which are associated The nervous system is traditionally divided into central with short-term mortality, the vast majority of peripheral nervous system (CNS) and peripheral nervous system (PNS) neuropathies seen in the emergency department (ED) are components. The PNS can be further subdivided into 12 subacute or chronic and are associated not with mortality but cranial and 31 spinal nerves. Disorders of the cranial nerves with long-term morbidity. are discussed in Chapter 103. Because diseases of the neuro- Current estimates suggest that about 1.5% of the U.S. popu- muscular junction and the myopathies are located distal to the lation suffers from peripheral neuropathy.2 Over 7% of the neuron itself, they are also considered separately in Chapter population has diabetes mellitus, with a prevalence rate of 104. Radiculopathies, which are disorders of the roots of the 20% in individuals older than 60 years. Roughly 50% of these PNS, are so commonly associated with musculoskeletal neck individuals have peripheral neuropathy.3 and back pain that they are mentioned only briefly here and are discussed in detail in Chapter 51. The simplest approach to diseases of the PNS parallels the CNS model of separating focal from nonfocal disease. In the ■ PRINCIPLES OF DISEASE PNS, the first broad category is the focal group, which can be Anatomy divided into those with evidence of single versus multiple lesions of peripheral nerves, known respectively as simple The spinal component of the PNS is shown schematically in mononeuropathies and multiple mononeuropathies (or mononeurop- Figure 105-2. -

Diagnostic Approach to Pulmonary Embolism and Lessons from a Busy Acute Assessment Unit in the UK M.M.A
Diagnostic approach to pulmonary embolism and lessons from a busy acute assessment unit in the UK M.M.A. Hamad1 P. Bhatia2 E. Ellidir1 The diagnosis of pulmonary embolism (PE) can be very elusive and, if missed,mayhave 2 fatal consequences. Conversely, PE can be over-diagnosed, with the concomitant risks M.M. Abdelaziz V. Connolly1 associated with unnecessary anticoagulation. Although there are many tests that used in the diagnosis of PE, no test can exclude this condition with 100% certainty,andPEhas 1Dept of Acute Medicine, James been reported even after a negative pulmonary angiography. The diagnosis of PE Cook University Hospital, depends on the interpretation of the available tests in the context of pre-test clinical Middlesbrough, UK probabilities. Ventilation/perfusion (V9/Q9) scan and computerised tomographic 2Dept of Medicine, Tameside pulmonary angiography (CTPA) are the main screening tests used for patients with Hospital, Ashton-under-Lyne, UK suspected PE. However, both V9/Q9 scan and CTPA have to be supplemented by other diagnostic modalities because of their diagnostic limitations. This article reviews the Correspondence M.M. Abdelaziz literature concerning the diagnosis of PE, with particular reference to the approach in our Dept of Medicine acute assessment unit. We conclude by describing two learning points from real cases Tameside Hospital presenting with suspected PE, in order to highlight how the diagnosis can bemissedor Ashton-under-Lyne, OL6 9RW, UK, made inaccurately. [email protected] Despite many diagnostic modalities, the presence or absence of an alternative diag- diagnosis of pulmonary embolism (PE) nosis which would explain the clinical remains very challenging, and PE can be presentation. -

Diagnosis of Pulmonary Embolism
This material is protected by U.S. copyright law. Unauthorized reproduction is prohibited. To purchase quantity reprints, please e-mail [email protected] or to request permission to reproduce multiple copies, please e-mail [email protected]. DIAGNOSTIC REASONING CATHERINE C. BURKE, MS, APRN, BC, ANP, AOCN®—ASSOCIATE EDITOR Diagnosis of Pulmonary Embolism Lynn M. Cloutier, RN, MSN, ACNP, AOCN® Case 1: A 77-year-old Caucasian woman with a probable diagnosis of ovarian cancer developed an acute onset of short- ness of breath two days after being discharged for a workup of symptomatic ascites. Her medical history was signifi cant for hypertension and atrial fi brillation, and she was taking therapeutic doses of coumadin. She presented to the emergency department; was found to have a large, left pleural effusion; and was admitted to the hospital. A thoracentesis removed 2.1 L of fl uid. The patient’s respiratory distress improved; however, she experienced a second episode of sudden onset shortness of breath prior to her anticipated discharge. She was afebrile, with a room air pulse oximetry of 80%, pulse of 121 beats per minute, and respiratory rate of 28. A chest x-ray (CXR) showed only a small pleural effusion remaining on the left. An electrocardiogram (EKG) showed atrial fi brillation. A chest computed tomography (CT) was performed and showed bilateral pulmonary emboli. Case 2: A 57-year-old, obese, African patient’s hospitalization, she developed a abdomen and pelvis was performed to American woman with newly diagnosed rapid heart rate of 139 beats per minute. delineate the extent of the abscess and endometrial cancer presented to clin- An EKG showed sinus tachycardia, and a to reassess the initial abscess.