Lecture 3 Acid Catalyzed Reactions I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lewis Acid Mediated Reactions of Olefins with Carbonyls
Lewis Acid Mediated Reactions of Olefins with Carbonyls Submitted by Stephen Flower For the Degree of PhD Of the University of Bath 2002 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be published without the prior written consent of the author. This thesis may be made available for consultation within the University Library and may be photocopied or lent to other libraries for the purposes of consultation. …………………………..(signed) …………………………..(date) Abstract This thesis is divided into 5 chapters. The first chapter reviews the literature of Carbonyl-ene and Prins reactions. Recent advances in these areas and their application to natural product synthesis and other targeted syntheses are discussed. The second chapter discusses the concept of desymmetrisation using selected examples, including desymmetrising Carbonyl-ene reactions. Chapter Three introduces and discusses the work undertaken in studying the desymmetrising intramolecular carbonyl-ene of a meso-dialdehyde. Chapter Four details the concept of pyruvate-Prins cyclisation giving examples of its potential use. The chapter also gives examples of the use of rigid stereodefined scaffolds and their application. The chapter describes the reactivity of substituted pyruvate esters with cyclic enol ethers, and the elaboration of the cyclised products. The fifth chapter provides experimental details of the procedures reported. i Acknowledgements Many thanks to Dr Mike Willis for all his help and guidance and boundless enthusiasm over the years; and for a detailed investigation of the local pubs. -

BI(III) Initiated Cyclization Reactions and Iodonium Fragmentation Kinetics
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2005 BI(III) Initiated Cyclization Reactions and Iodonium Fragmentation Kinetics Yajing Lian College of William & Mary - Arts & Sciences Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Inorganic Chemistry Commons Recommended Citation Lian, Yajing, "BI(III) Initiated Cyclization Reactions and Iodonium Fragmentation Kinetics" (2005). Dissertations, Theses, and Masters Projects. Paper 1539626842. https://dx.doi.org/doi:10.21220/s2-zj7g-fp23 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. BI(III) INITIATED CYCLIZATION REACTIONS AND IODONIUM FRAGMENTATION KINETICS A Thesis Presented to The Faculty of the Department of Chemistry The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Science by Yajing Lian 2005 APPROVAL SHEET This thesis is submitted in partial fulfillment of The requirements for the degree of Master of Science A Yajing Lian Approved by the Committee, December 20, 2005 Robert J. Hinkle, Chair Christopher Jl A] Robert D. Pike TABLE OF CONTENTS Page Acknowledgements VI List of Tables Vll List of Schemes V lll List of Figures IX Abstract XI Introduction Chapter I Secondary -

Abstract Multicomponent Reactions of Salicylaldehyde, Cyclic Ketones, and Arylamines Through Cooperative Enamine-Metal Lewis
ABSTRACT MULTICOMPONENT REACTIONS OF SALICYLALDEHYDE, CYCLIC KETONES, AND ARYLAMINES THROUGH COOPERATIVE ENAMINE-METAL LEWIS ACID CATALYSIS by Ryan Gregory Sarkisian Multicomponent reactions (MCRs) are the most atom economic, highly selective, and convergent type of reaction. This allows for a reaction to have a wide scope and allows for maximization of the complexity of a product. Catalyzing these MCRs with asymmetric catalysis is a novel way to introduce stereocontrol into highly complex molecules with various functional groups. Asymmetric catalysis is considered the most efficient method for constructing highly functionalized optically active stereopure compounds. There are three pillars of asymmetric catalysis: biocatalysis, transition metal catalysis, and organocatalysis. This research focuses on two of these pillars, transition metal catalysis and organocatalysis, working cooperatively to catalyze this MCR. The focus is to educate or refresh the audience on the basic topics that make up the complexity of the MCRs being catalyzed by cooperative asymmetric catalysis. Ultimately to explore the cooperative catalysts used to synthesize both the racemic and asymmetric three fused ring products (9-((4-methoxyphenyl)amino)-2,3,4,4a,9,9a-hexahydro-1H-xanthen-4a-ol). MULTICOMPONENT REACTIONS OF SALICYLALDEHYDE, CYCLIC KETONES, AND ARYLAMINES THROUGH COOPERATIVE ENAMINE-METAL LEWIS ACID CATALYSIS A THESIS Submitted to the Faculty of Miami University in partial fulfillment of the requirements for the degree of Master of Science Department of -

Catalytic Direct Asymmetric Michael Reactions
ORGANIC LETTERS 2001 Catalytic Direct Asymmetric Michael Vol. 3, No. 23 Reactions: Taming Naked Aldehyde 3737-3740 Donors Juan M. Betancort and Carlos F. Barbas III* The Skaggs Institute for Chemical Biology and the Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 [email protected] Received September 5, 2001 ABSTRACT Direct catalytic enantio- and diastereoselective Michael addition reactions of unmodified aldehydes to nitro olefins using (S)-2-(morpholinomethyl)- pyrrolidine as a catalyst are described. The reactions proceed in good yield (up to 96%) in a highly syn-selective manner (up to 98:2) with enantioselectivities approaching 80%. The resulting γ-formyl nitro compounds are readily converted to chiral, nonracemic 3,4-disubstituted pyrrolidines. The Michael reaction is generally regarded as one of the Typically, carbon nucleophiles that contain an active most efficient carbon-carbon bond forming reactions, and methylene center such as malonic acid esters, â-keto esters, studies concerning this reaction have played an important nitroalkanes, etc. have been studied in the Michael reaction. role in the development of modern synthetic organic Carbonyl compounds, and ketones in particular, have gener- chemistry.1 As the demand for optically active compounds ally only been used as donors following their preactivation has soared in recent years, much progress has been made in by conversion into a more reactive species such as enol or the development of asymmetric variants of this reaction, enamine equivalents.5,6 In these cases, additional synthetic providing for the preparation of Michael adducts with high enantiomeric purity.2 Though remarkable advances have been (3) (a) Chataigner, I.; Gennari, C.; Ongeri, S.; Piarulli, U.; Ceccarelli, S. -

Study of Reactions of Two Mannich Bases Derived of 4’-Hydroxychalcones with Glutathione by RP‑TLC, RP‑HPLC and RP‑HPLC‑ESI‑MS Analysis
http://dx.doi.org/10.21577/0103-5053.20160260 J. Braz. Chem. Soc., Vol. 28, No. 6, 1048-1062, 2017. Printed in Brazil - ©2017 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00 Article Study of Reactions of Two Mannich Bases Derived of 4’-Hydroxychalcones with Glutathione by RP-TLC, RP-HPLC and RP-HPLC-ESI-MS Analysis Aline Bernardes,a,b Caridad N. Pérez,a Mátyás Mayer,c Cameron C. da Silva,a Felipe T. Martinsa and Pál Perjési*,b aInstituto de Química, Universidade Federal de Goiás, 74690-900 Goiânia-GO, Brazil bInstitute of Pharmaceutical Chemistry and cDepartment of Forensic Medicine, University of Pécs, H-7624 Pécs, Hungary 4’-Hydroxychalcones have been reported to possess several beneficial biological effects. Several lines of evidence accumulated to demonstrate increased biological activities of the Mannich base derivatives of the parent 4’-hydroxychalcones. Bioactivities of chalcones and related α,β-unsaturated ketones are frequently associated with their reactivity with cellular thiols, such as GSH. For comparison of GSH reactivity, two bis Mannich bases of two 4’-hydroxychalcones were synthesized and reacted with GSH under non-cellular conditions. Reversed-phase thin layer chromatography (RP-TLC) and reversed-phase high performance liquid chromatography (RP-HPLC) analysis showed formation of two polar products which structures were confirmed by RP-HPLC-ESI-MS (RP-HPLC-electrospray ionization mass spectrometry) as 1:1 chalcone-GSH adducts in each case. At pH values below 8.0, the two bis Mannich bases showed higher GSH reactivity than two 4’-hydroxychalcones. Influence of the nature of the amino groups, the ring-B substituents and pH of the medium on reactivity was also investigated. -

Copyrighted Material
Index Abbreviations receptor sites 202, 211 weak 122 amino acids, Table 500–1 muscarinic 413 weak, pH calculation 147–8 nucleic acids 551 nicotinic 413 Acid–base nucleotides, Table 551 as quaternary ammonium salt 202 catalysis, enzymes 516 peptides and proteins 504 Acetylcholinesterase equilibria 121 phosphates and diphosphates 277 enzyme mechanism 519–21 interactions, predicting 155–7 structural, Table 14 hydrolysis of acetylcholine 279 Acidic reagents, Table 157 ACE (angiotensin-converting enzyme) inhibitors 279 Acidity enzyme action 532 Acetyl-CoA carboxylase, in fatty acid acidity constant 122 inhibitors 532 biosynthesis 595 bond energy effects 125 Acetal Acetyl-CoA (acetyl coenzyme A) definition 121 in etoposide 233 as acylating agent 262 electronegativity effects 125 formation 229 carboxylation to malonyl-CoA 595, hybridization effects 128 polysaccharides as polyacetals 232 609 inductive effects 125–7 as protecting group 230 Claisen reaction 381 influence of electronic and structural Acetal and ketal enolate anions 373 features 125–34 cyclic, as protecting groups 481 in Krebs cycle 585 and leaving groups, Table 189 groups in sucrose 231 from β-oxidation of fatty acids 388 pKa values 122–5 Acetaldehyde, basicity 139 as thioester 262, 373 resonance / delocalization effects 129–34 Acetamide, basicity 139 Acetylene, bonding molecular orbitals 31 Acidity (compounds) Acetaminophen, see paracetamol Acetylenes, acidity 128 acetone 130 Acetoacetyl-CoA, biosynthesis from N-Acetylgalactosamine, in blood group acetonitrile 365 acetyl-CoA 392 -

Accessing Light-Sensitive Polycyclic Aromatics Through an Alkynyl-Prins Cyclization
W&M ScholarWorks Undergraduate Honors Theses Theses, Dissertations, & Master Projects 4-2018 Accessing Light-Sensitive Polycyclic Aromatics through an Alkynyl-Prins Cyclization Daniel John Speer Follow this and additional works at: https://scholarworks.wm.edu/honorstheses Part of the Chemistry Commons Recommended Citation Speer, Daniel John, "Accessing Light-Sensitive Polycyclic Aromatics through an Alkynyl-Prins Cyclization" (2018). Undergraduate Honors Theses. Paper 1244. https://scholarworks.wm.edu/honorstheses/1244 This Honors Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Undergraduate Honors Theses by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Accessing Light-Sensitive Polycyclic Aromatics through an Alkynyl-Prins Cyclization A thesis submitted in partial fulfillment of the requirement for the degree of Bachelor of Science with Honors in the Department of Chemistry from the College of William and Mary Accepted for Dr. Jonathan Scheerer Dr. Christopher Abelt Dr. Anne Rasmussen Directed by Dr. Robert J. Hinkle By Daniel John Speer April 26, 2018 Table of Contents Acknowledgements 3 Abstract 4 List of Figures 5 Introduction 6 Prins Chemistry and the alkynyl-Prins Cyclization 6 Photochromic Compounds 9 Cannabinoids 12 Results and Discussion 16 Development of Synthetic Procedure 16 Synthesis of Starting Material 17 Proposed Mechanism for the Cyclization 21 Synthesis and NMR Properties of Chromene Products 24 X-ray Crystallographic Analysis 27 UV/Visible Spectrophotometric Analysis 30 Conclusion 33 Experimental Methods 34 Materials and Methods 34 Synthetic Procedures 35 References 43 Supporting Information 46 Mass Spectrum of 11 46 X-ray Crystal Data and Structure Refinement for 11 47 2 Acknowledgements I would like to thank everyone involved in this process. -

Synthetic Strategies for the Lepadiformines and Cylindricine C Via Tandem Schmidt Reactions
Synthetic Strategies for the Lepadiformines and Cylindricine C via Tandem Schmidt Reactions By Angelica M. Meyer Submitted to the graduate degree program in the Medicinal Chemistry and the Graduate Faculty of The University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ________________________________ Chairperson: Jeffrey Aubé ________________________________ Dr. Thomas E. Prisinzano ________________________________ Dr. Ryan A. Altman ________________________________ Dean Kenneth L. Audus ________________________________ Dr. Paul R. Hanson Date Defended: December 5, 2011 The Dissertation Committee for Angelica M. Meyer certifies that this is the approved version of the following thesis: Synthetic Strategies for the Lepadiformines and Cylindricine C via Tandem Schmidt Reactions ________________________________ Chairperson Jeffrey Aubé Date approved: December 5, 2011 ii Abstract Marine flora and fauna have provided numerous alluring natural products, some of which contribute to treating diseases. The genus Clavelina is the source of a variety of tricyclic alkaloids, including the lepadiformine and cylindricine families. Novel approaches to synthesizing these molecules are sought after to increase their accessibility and for analogue development. In this dissertation, reaction sequences involving an intramolecular Schmidt transformation, which can quickly build up the molecular architecture associated with these targets is described. In one approach, the Lewis acid promoted intramolecular Schmidt reaction is combined in series with a Prins reaction to afford an interesting tricyclic lactam. This methodology culminates in a formal synthesis of lepadiformine A and a total synthesis of lepadiformine C. In another project, a tandem Diels–Alder/Schmidt reaction is utilized to prepare a similar tricyclic lactam. This process is applied toward an asymmetric total synthesis of (–)-cylindricine C. -

Generation of a Structurally Diverse Library Through Alkylation and Ring
70 Acta Chim. Slov. 2013, 60, 70–80 Scientific paper Generation of a Structurally Diverse Library through Alkylation and Ring Closure Reactions Using 3-Dimethylamino-1-(thiophen-2-yl)propan-1-one Hydrochloride* Gheorghe Roman Petru Poni Institute of Macromolecular Chemistry, 41A Aleea Gr. Ghica Vodâ, Ias¸i 700487, Romania Corresponding author: E-mail: [email protected] Received: 01-06-2012 Abstract 3-Dimethylamino-1-(thiophen-2-yl)propan-1-one hydrochloride (2), a ketonic Mannich base derived from 2-acetyl- thiophene, was used as a starting material in different types of alkylation and ring closure reactions with a view to gene- rate a structurally diverse library of compounds. Compound 2 reacts with S-alkylated dithiocarbamic acid salts and aryl mercaptans to produce dithiocarbamates and thioethers, respectively. The dimethylamino moiety in compound 2 was exchanged with various aliphatic secondary and aromatic primary and secondary amines, whereas monocyclic NH-azoles such as pyrazole, imidazole, 1,2,4-triazole, and tetrazole were N-alkylated by compound 2. Ketones, pyrrole and in- doles have been the substrates subjected to C-alkylation reactions by compound 2. Ring closure reactions of compound 2 with a suitable bifunctional nucleophile yielded pyrazolines, pyridines, 2,3-dihydro-1,5-1H-benzodiazepines, 2,3- dihydro-1,5-1H-benzothiazepine, pyrimido[1,2-a]benzimidazole and 4-hydroxypiperidine derivatives. Keywords: Ketonic Mannich base, alkylation, amine exchange, cyclization. 1. Introduction through the replacement of the easily leaving dimethyla- mino group by various nucleophiles. Also, several cycliza- The chemistry of Mannich bases has drawn a great tions of the aforementioned Mannich base with bifunctio- deal of attention owing to the high synthetic potential and nal nucleophiles to 5-, 6- and 7-membered nitrogen-con- the outstanding applications of this class of compounds.2–4 taining heterocycles have been investigated. -

Comparative Total Syntheses of Strychnine
Comparative Total Syntheses of Strychnine N C D MacMillan Group Meeting N H O H A H B E Nathan Jui H N N H H F G July 22, 2009 O O O References: Pre-Volhardt: Bonjoch Chem. Rev. 2000, 3455. Woodward Tetrahedron, 1963, 247. Volhardt J. Am. Chem. Soc. 2001, 9324. Magnus J. Am. Chem. Soc. 1993, 8116. Martin J. Am. Chem. Soc. 2001, 8003. Overman J. Am. Chem. Soc. 1995, 5776. Bodwell Angew. Chem. Int. Ed. 2002, 3261. Kuehne J. Org. Chem. 1993, 7490. Mori J. Am. Chem. Soc. 2003, 9801. Kuehne J. Org. Chem. 1998, 9427. Shibasaki Tetrahedron 2004, 9569. Rawal J. Org. Chem. 1994, 2685. Fukuyama J. Am. Chem. Soc. 2004, 10246. Bosch, Bonjoch Chem. Eur. J. 2000, 655. Padwa Org. Lett. 2007, 279. History and Structure of (!)-Strychnine ! Isolated in pure form in 1818 (Pelletier and Caventou) ! Structural Determination in 1947 (Robinson and Leuchs) ! 24 skeletal atoms (C21H22N2O2) ! Isolated in pure form in 1818 (Pelletier and Caventou) N ! Over !25 07 -pruinbglisc,a 6ti-osntesr epoecrteaninteinrgs to structure ! Structural Determination in 1947 (Robinson and Leuchs) ! Notor!io u Ss ptoirxoicne (nletethr a(lC d-o7s) e ~10-50 mg / adult) N H H ! Over 250 publications pertaining to structure O O ! Comm!o nClyD uEs eridn gr osdyesntet mpoison ! Notorious poison (lethal dose ~10-50 mg / adult) ! Hydroxyethylidine Strychnos nux vomica ! $20.20 / 10 g (Aldrich), ~1.5 wt% (seeds), ~1% (blossoms) "For it's molecular size it is the most complex substance known." -Robert Robinson History and Structure of (!)-Strychnine ! 24 skeletal atoms (C21H22N2O2) N ! 7-rings, 6-stereocenters ! Spirocenter (C-7) N H H O O ! CDE ring system ! Hydroxyethylidine "For it's molecular size it is the most complex substance known." -Robert Robinson "If we can't make strychnine, we'll take strychnine" -R. -
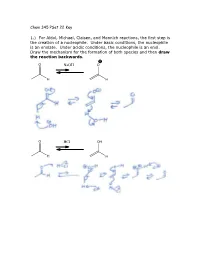
Chem 345 Pset 22 Key 1.) for Aldol, Michael, Claisen, and Mannich
Chem 345 PSet 22 Key 1.) For Aldol, Michael, Claisen, and Mannich reactions, the first step is the creation of a nucleophile. Under basic conditions, the nucleophile is an enolate. Under acidic conditions, the nucleophile is an enol. Draw the mechanism for the formation of both species and then draw the reaction backwards. O NaOH O H H O HCl OH H H 2.) In the case of a Mannich reaction, the electrophile (an imine) must also be made. Draw the mechanism for the forward and backward reaction: O MeNH2 N H H cat. AcOH H H 3.) When a secondary amine is used and the carbonyl has an enolizable proton, an enamine can be formed. Are enamines nucleophilic or electrophilic? Nucleophilic H O N N H2O H cat. AcOH H enamine N N H H enamine 4.) The following reaction is the Lobry-de-Bruyn-von-Ekenstein reaction. Don’t worry about the name. I only recently found out that that was the official name for the rearrangement leading from glucose to fructose or mannose and back again. I was taught it under a different name: the Carbohydrate Game. Under acidic or basic conditions, it is possible that the following sugars (glucose, fructose, and mannose) are in equilibrium with each other. Draw the acid catalyzed version and the base catalyzed version. (Hint: this is just a repeat of question 1.) O O H H H2C OH + + HO H H3O H OH H3O O or or HO- HO- HO H HO H HO H H OH H OH H OH H OH H OH H OH H2C OH H2C OH H2C OH D-glucose D-fructose D-mannose O O H H H2C OH + + HO H H3O H OH H3O O or or HO- HO- HO H HO H HO H H OH H OH H OH H OH H OH H OH H2C OH H2C OH H2C OH D-glucose D-fructose D-mannose The sugars are drawn in a Fisher projection (or what I think of is a dead cat projection). -

Heterocyclic Chemistrychemistry
HeterocyclicHeterocyclic ChemistryChemistry Professor J. Stephen Clark Room C4-04 Email: [email protected] 2011 –2012 1 http://www.chem.gla.ac.uk/staff/stephenc/UndergraduateTeaching.html Recommended Reading • Heterocyclic Chemistry – J. A. Joule, K. Mills and G. F. Smith • Heterocyclic Chemistry (Oxford Primer Series) – T. Gilchrist • Aromatic Heterocyclic Chemistry – D. T. Davies 2 Course Summary Introduction • Definition of terms and classification of heterocycles • Functional group chemistry: imines, enamines, acetals, enols, and sulfur-containing groups Intermediates used for the construction of aromatic heterocycles • Synthesis of aromatic heterocycles • Carbon–heteroatom bond formation and choice of oxidation state • Examples of commonly used strategies for heterocycle synthesis Pyridines • General properties, electronic structure • Synthesis of pyridines • Electrophilic substitution of pyridines • Nucleophilic substitution of pyridines • Metallation of pyridines Pyridine derivatives • Structure and reactivity of oxy-pyridines, alkyl pyridines, pyridinium salts, and pyridine N-oxides Quinolines and isoquinolines • General properties and reactivity compared to pyridine • Electrophilic and nucleophilic substitution quinolines and isoquinolines 3 • General methods used for the synthesis of quinolines and isoquinolines Course Summary (cont) Five-membered aromatic heterocycles • General properties, structure and reactivity of pyrroles, furans and thiophenes • Methods and strategies for the synthesis of five-membered heteroaromatics