(12) Patent Application Publication (10) Pub. No.: US 2016/0114338 A1 Snead (43) Pub
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Petition to the Administrative Council of the European Patent Organization
August 10, 2019 BY EMAIL Petition to the Administrative Council of the European Patent Organization Cc: Antonio Campinos, President of the EPO Emmanuel Macron, President, French Republic Christoph Ernst, VP Legal/ International Affairs Angela Merkel, Chancellor, Germany Karin Seegert, COO, Healthcare & Chemistry Mark Rutte, Prime Minister, The Netherlands Piotr Wierzejewski, Quality Management Boris Johnson, Prime Minister, United Kingdom Stoyan Radkov - Applicant’s Representative Cornelia Rudloff-Schäffer, President, German PTO Tim Moss, CEO, Intellectual Property Office of Cadre Philippe, Director, French National Institute the United Kingdom of Industrial Property Vincentia Rosen-Sandiford, Director, Netherlands Patent Office Re: European patent application 09735962.4; and European divisional application 17182663.9; Applicant: Asha Nutrition Sciences, Inc. Dear Delegates in the Administrative Council, We have been prosecuting the referenced patent applications directed to critical innovations for public health at EPO for last 10 years. However, rather than advancing the innovations EPO has been obstructing them. EPO statements in the prosecution history evidence that rejections have been applied to oblige us to reduce the claimed scope, even though as per provisions of European Patent Convention, the subject claims are perfectly patentable. A narrow patent is not synonymous with a quality patent. The metric of quality disregarded by EPO is genuine innovation, measured by betterment of life achieved, though that is the very purpose of patents and is built into the law. For example, solutions to critical unmet needs are inventive even if claims are otherwise obvious (GL1, G-VII, 10.3). Narrow patents in the nutrition arts have already caused great harm to public health and created patent-practice- made humanitarian crises by creating misinformation and taken us farther away from solving nutritional problems, preventative solutions, and sustainability. -

FINAL COPY Swietlana Borkowska Final
BIODIESEL POTENTIAL IN ICELAND Swietlana Borkowska A 30 credit units Master´s thesis Supervisor Asgeir Ivarsson A Master’s thesis done at RES │ the School for Renewable Energy Science in affiliation with University of Iceland & the University of Akureyri Akureyri, February 2009 1 ‘Biodiesel Potential in Iceland’ A 30 credit units Master’s thesis © Swietlana Borkowska, 2009 RES │ the School for Renewable Energy Science Solborg v/Nordurslod 600 Akureyri, Iceland Telephone + 354 464 0100 Printed at Stell Akureyri, Iceland 2009 2 ABSTRACT The importance of increasing the global share of biofuels in transportation goes without saying. Iceland, where the consumption of fossil fuels is considerable, has a viable potential for introducing biodiesel in its otherwise exceptional renewable overall energy portfolio. In this study, a full picture of the possibilities of biodiesel production in Iceland was provided. After the theoretical introduction of all major aspects of a biodiesel economy, an assessment of its applicability in Iceland was performed. A survey of potential feedstocks was performed. It was concluded that in a short term perspective, a small scale production (300-2,000 tons/yr) can be carried out using domestically available waste raw material, and full scale production (15,000-80,000 tons/yr) will depend on imported feedstock. After laboratory research, including waste vegetable oil (WVO), the main domestic feedstock currently available, the recommendation for the production process of a small production plant was made. It includes acid esterification of free fatty acids (FFA) followed by alkali transesterification and methanol recovery from the reacted mixture. At this stage, distillation of crude FAME was suggested, however further research is necessary. -

Niop Trading Rules
NIOP TRADING RULES Effective July 2013 Minor changes and corrections (approved by the NIOP Technical Committee) have been included as of July 29, 2013 APPLICATION OF RULES INCORPORATION OF THESE RULES OR ANY PORTION THEREOF IS NOT MANDATORY BUT IS OPTIONAL BETWEEN PARTIES TO CONTRACTS. Published by the National Institute of Oilseed Products 750 National Press Building, 529 14th St NW Washington, D.C. 20045 TEL: (202) 591-2438 FAX: (202) 591-2445 e-mail: [email protected] Internet: www.niop.org ©2013 by the National Institute of Oilseed Products NIOP TRADING RULES TABLE OF CONTENTS CHAPTER 1 - TYPES OF SALES RULE 1.1 TRADE PRACTICE 1 -1 1.2 PLACE OF CONTRACT 1 -1 1.3 C.I.F. (COST, INSURANCE AND FREIGHT) 1 -1 LISTING OF DOCUMENTS 1 -2 1.4 C.& F. (COST AND FREIGHT) 1 -3 1.5 F.O.B. VESSEL (FREE ON BOARD VESSEL) 1 -3 1.6 F.A.S. VESSEL (FREE ALONGSIDE) -NAMED PORT OF SHIPMENT 1 -5 1.7 EX DOCK (NAMED PORT OF IMPORTATION) 1 -6 1.8 EX WAREHOUSE 1 -7 1.9 MISCELLANEOUS TYPES OF SALES 1 -7 1.10 VESSEL CLASSIFICATION 1 -7 1.11 PUBLIC HEALTH SECURITY AND BIOTERRORISM PREPAREDNESS 1 -7 CHAPTER 2 - SHIPMENT RULE 2.1 TIME 2 -1 2.2 DAYS OR HOURS 2 -1 2.3 NOTICE 2 -1 2.4 TENDERS 2 -1 2.5 EXTENSION OF SHIPMENT 2 -1 2.6 PROOF OF ORIGIN 2 -3 2.7 TRANSHIPMENT 2 -3 2.8 SHIPPING INSTRUCTIONS 2 -3 2.9 VESSEL NOMINATION AND DECLARATION OF DESTINATION 2 -3 2.10 BILL OF LADING-EVIDENCE OF DATE OF SHIPMENT 2 -3 CHAPTER 3 - TANK CARS, TRUCKS, BARGES AND CONTAINERS RULE 3.1 DATE OF SHIPMENT 3 -1 3.2 TIME OF SHIPMENT 3 -1 3.3 SPREAD (SCATTERED) DELIVERIES OF SHIPMENTS 3 -1 3.4 F.O.B. -

Effects of Different Nitrogen Sources and Doses of Application on Growth and Active Constituents of Cynara Cardunculus L
250 Middle East Journal of Agriculture Research, 3(2): 250-262, 2014 ISSN 2077-4605 Effects of Different Nitrogen Sources and Doses of Application on Growth and Active Constituents of Cynara cardunculus L. plants. 1Atef Z. Sarhan, 2Hend E. Wahba,1Amal A. Nasr,2Adel B. Salama, 2Heba M. Gad 1Ornamental Horticulture Dept., Faculty of Agriculture, Cairo University, Giza, Egypt. 2Medicinal and Aromatic Plants Research Dept., Pharmaceutical and Drug Industries Division, National Research Centre. Dokki, Egypt. ABSTRACT Cynara cardunculus L. have been known for long time as a medicinal plant for treatments of many diseases but its agricultural studies and chemical composition did not have enough researches in Egypt. Two field experiments were carried out at the Experimental Farm of Agriculture Faculty, Cairo University during two successive seasons 2009/2010 and 2010/2011, to study the effect of nitrogen dressing application (Urea, ammonium nitrate and ammonium sulphate) at different doses (0,40, 60 and 80 N unit/fed) on growth, yield parameters and chemical composition plants. The results showed that application of all nitrogen forms and doses and their interaction significantly increased the growth characteristics and chemical composition of Cynara cardunculus as compared to untreated plants. The best treatment was ammonium nitrate at 60 N unit/fed which increased quantitative and qualitative plant production. Key words: Cynarac cardunculus, Polyphenols, Flavonoids, chlorogenic acid, Nitrogen, lipids, Fertilization. Introduction Cynara cardunculus L. is a cross–pollinated and highly heterozygous plant belonging to the family Asteraceae (Compositae), is an herbaceous perennial crop commonly named cardoon. This plant is used worldwide and represents a notable ingredient of the Mediterranean diet (Fratianniet al., 2007). -

The Soap Making Companion a Guide for Beginners 2Nd Edition
The Soap Making Companion A Guide for Beginners 2nd Edition The companion guidebook to the Thermal Mermaid Artisan Soap Makers Course By Thermal Mermaid & Jennifer Tynan Copyright 2020 by SpeckledEggPublishing All rights reserved. This book, or parts within may not be reproduced without permission from the publisher. Published by SpeckledEggPublishing Waterbury, CT 06706 https://speckledeggpublishing.com Contains material edited and modified from The Soap Making Companion: A Guide for Beginners, 1st Edition, by Jennifer Tynan, copyright 2018, 2015. ISBN: 9798643284789 Printed in the United States of America This publication is designed to provide accurate information in respect to the material presented. It is sold with the understanding that the recipes and suggestions are personal thoughts and understanding on the artisan craft of soap making and is not engaged in professional or medical advice. No claims are made about any of the recipes regarding cures, therapies, or treatments for personal care. Soap is made for cleaning, and the recipes in this book are geared toward the artistic techniques in creating only soap. Photography by Jennifer Tynan 10 9 8 7 6 5 4 3 2 2 Table of Contents Introduction 1. 1: Soaping, Setup, & Safety Welcome to the Beginning Gather the Supplies You Need Lye Safety & First Aid How to Mix & Use Lye Properly Saponification & Lye Calculations by Hand First Simple Soap Recipe 2. Cold Process Method, Techniques & Designs White Soap Trace Lavender Flowers Gel Insulating Glycerin Rivers Soda Ash Neapolitan Clay Bar Salt Bar & Brine Drop Swirl Soap 3 Hangar Swirl In the Bowl Swirl Column Pour Peacock Swirl Lava Bubbles 3. -
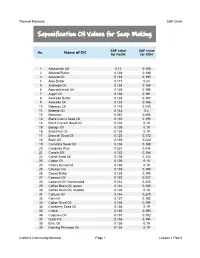
Saponification Oil Values for Soap Making
Thermal Mermaid SAP Chart Saponification Oil Values for Soap Making SAP value SAP value No. Name of Oil for NaOH for KOH 1 Abyssinian Oil 0.12 0.168 2 Almond Butter 0.134 0.188 3 Almond Oil 0.139 0.195 4 Aloe Butter 0.171 0.24 5 Andiroba Oil 0.134 0.188 6 Apricot Kernal Oil 0.139 0.195 7 Argan Oil 0.136 0.191 8 Avocado Butter 0.133 0.187 9 Avocado Oil 0.133 0.186 10 Babassu Oil 0.175 0.245 11 Baobab Oil 0.143 0.2 12 Beeswax 0.067 0.094 13 Black Cumin Seed Oil 0.139 0.195 14 Black Currant Seed Oil 0.135 0.19 15 Borage Oil 0.135 0.19 16 Brazil Nut Oil 0.135 0.19 17 Broccoli Seed Oil 0.123 0.172 18 Buriti Oil 0.159 0.223 19 Camelina Seed Oil 0.134 0.188 20 Candelila Wax 0.031 0.044 21 Canola Oil 0.133 0.186 22 Carrot Seed Oil 0.103 0.144 23 Castor Oil 0.128 0.18 24 Cherry Kernal Oil 0.135 0.19 25 Chicken Fat 0.139 0.195 26 Cocoa Butter 0.138 0.194 27 Coconut Oil 0.183 0.257 28 Coconut Oil, fractionated 0.232 0.325 29 Coffee Been Oil, green 0.132 0.185 30 Coffee Bean Oil, roasted 0.128 0.18 31 Cohune Oil 0.146 0.205 32 Corn Oil 0.137 0.192 33 Cotton Seed Oil 0.138 0.194 34 Cranberry Seed Oil 0.135 0.19 35 Crisco 0.138 0.193 36 Cupuacu Oil 0.137 0.192 37 Duck Fat 0.138 0.194 38 Emu Oil 0.135 0.19 39 Evening Primrose Oil 0.135 0.19 Crafter's Community Member Page 1 Lesson 1 Part 5 Thermal Mermaid SAP Chart SAP value SAP value No. -

The Belize Ag Report Belize’S Most Complete Independent Agricultural Publication
The Belize Ag Report Belize’s most complete independent agricultural publication pg. 16 NOV 2013 - JAN 2014 BGGA on Corn pg. 17 ISSUE 23 Thiessen Liquid Fertilizer Rice Field Day Pg. 12 Field Margins pg. 18 Mamey Sapote pg. 22 Syngenta.com Panela pg. 21 Citrus Industry pg. 5 Cohune Stove pg. 3 57 ac Farm 668-0749 / 663-6777 holdfastbelize.com US$ 275 K FARM LISTINGS Home with Pool See pg. 2 & 26 www.brandbelize.com NOV 2013 - JAN 2014 BelizeAgReport.com 1 Harvesting Ag News from All of Belize Land is our language TM We speak farm land and river land We are involved in agriculture so we are familiar with local issues and understand agricultural concerns. We have buyers/renters for farmland. Let us know if have land to sell or rent. S L As a small company, we personalize our service to both buyer and seller. Court Roberson•Beth Roberson 668-0749 / 663-6777 www.holdfastbelize.com Belize Spice Farm && Botanical Gardens Come and visit the largest Vanilla & Black Pepper farm in Belize!!! Come enjoy our tropical plant collection which in addition to Vanilla and Black Pepper, includes Cardamom, Clove, Nutmeg, Cashew, Rambuttan, Sapote, Anjili, Bilimbi, Carambola, Nellipuli, Jackfruit, Mangos, Jatropha and many flowering plants too many to list! Tours are open to the public!!! Nursery plants on sale til end of Chocolate Festival May 26th Golden Stream, Southern Highway, Toledo District 221 km, or approximately 3 hours drive from Belize City (501) 732 - 4014 • [email protected] www.belizespicefarm.com NOV 2013 - JAN 2014 BelizeAgReport.com 2 Harvesting Ag News from All of Belize Belize’s ‘Green Coal’: cohune nuts. -

Securing Food and Livelihoods: Opportunities and Constraints to Sustainably Enhancing Household Food Production in Santa Familia Village, Belize
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 2014 SECURING FOOD AND LIVELIHOODS: OPPORTUNITIES AND CONSTRAINTS TO SUSTAINABLY ENHANCING HOUSEHOLD FOOD PRODUCTION IN SANTA FAMILIA VILLAGE, BELIZE Siobhan B. Lozada The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Lozada, Siobhan B., "SECURING FOOD AND LIVELIHOODS: OPPORTUNITIES AND CONSTRAINTS TO SUSTAINABLY ENHANCING HOUSEHOLD FOOD PRODUCTION IN SANTA FAMILIA VILLAGE, BELIZE" (2014). Graduate Student Theses, Dissertations, & Professional Papers. 4202. https://scholarworks.umt.edu/etd/4202 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. SECURING FOOD AND LIVELIHOODS: OPPORTUNITIES AND CONSTRAINTS TO SUSTAINABLY ENHANCING HOUSEHOLD FOOD PRODUCTION IN SANTA FAMILIA VILLAGE, BELIZE By SIOBHAN BURMA LOZADA Latin American Studies, University of California at San Diego, 2008 Thesis presented in partial fulfillment of the requirements for the degree of Master of Science in Resource Conservation, International Conservation and Development University of Montana Missoula, MT April 2014 Approved by: Sandy Ross, Dean of the Graduate School Graduate School Stephen F. Siebert, Chair Department of Forest Management Dane Scott, Co-chair Department of Society and Conservation Sarah J. Halvorson Department of Geography Joshua Slotnick Department of Environmental Studies © COPYRIGHT by Siobhan Burma Lozada 2014 All Rights Reserved ii ABSTRACT Rural households play an active role in reorganizing their livelihood strategies to respond to external stresses and shocks. -

RULE 5.12 - PRIOR CARGO LISTINGS September 1, 2007
RULE 5.12 - PRIOR CARGO LISTINGS September 1, 2007 NOTE: All substances on Acceptable Lists 1 and 2 also appear on the NIOP-FOSFA Harmonized International List of Acceptable Previous Cargoes except those designated by (*). Substances not appearing on either Acceptable List 1 or 2 are not permitted to be carried as the last cargo immediately prior to edible oils. 5.12.1 - ACCEPTABLE PRIOR CARGO - LIST NO. 1 The following items are acceptable prior cargoes for transported edible oils, which may or may not be further processed prior to use: CARGO COMMON NAME Acetic acid – (USP and Food Grades only) Alcoholic beverages (i.e. rum, wine) Almond oil Anchovy oil Apple juice concentrate Apricot kernel oil Avocado oil Babassu oil Beechnut oil Beeswax (white & yellow) Candelilla wax Canola oil - LEAR (“double zero”) Carnauba wax Castor oil Cocoa butter Coconut oil Cod liver oil Cod oil Cohune oil Corn oil - (maize oil) Corn syrup Cottonseed oil Dairy products (limited per USA 21CFR, Part 131) Dextrose solution Ethyl alcohol – (Food Grades only) Fish liver oil Fish oil Fructose Glucose syrup Glycerin Grape juice concentrate Grapeseed oil Hazelnut oil Herring oil Illipe butter - (mowrah butter) Juice concentrates (i.e. apple, grape) Lactic acid (limited per USA 21CFR, Parts 150 & 184) Lard Linseed oil Lycopersicum esculentum oil - (tomato seed oil) Menhaden oil Molasses Montan wax Murumuru fat Mustard seed oil Non-alcoholic beverages Nutmeg butter Olive oil Orange juice slurry Palm kernel oil Palm kernel olein Palm kernel stearin Palm oil 5.12.1 - -

Wo 2009/131939 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 29 October 2009 (29.10.2009) WO 2009/131939 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A23D 7/00 (2006.01) A61K 9/107 (2006.01) kind of national protection available): AE, AG, AL, AM, A23L 1/29 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, (21) International Application Number: EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, PCT/US2009/041 114 HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 20 April 2009 (20.04.2009) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, (25) Filing Language: English SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, (26) Publication Language: English UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/046,747 2 1 April 2008 (21 .04.2008) US kind of regional protection available): ARIPO (BW, GH, 61/075,708 25 June 2008 (25.06.2008) US GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, 61/1 11,593 5 November 2008 (05.1 1.2008) US ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, 12/426,034 17 April 2009 (17.04.2009) US TM), European (AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, (71) Applicant (for all designated States except US): ASHA MC, MK, MT, NL, NO, PL, PT, RO, SE, SI, SK, TR), LIPID SCIENCES, INC. -

Plant Resources of South-East Asia (PROSEA)
,. / UXVT/O.J _/ t //^ <P*)- ouj Plant Resources ofSouth-Eas t Asia No 14 Vegetable oils and fats H.A.M. van der Vossen and B.E. Umali (Editors) H Backhuys Publishers, Leiden 2001 \h^ i ^35^50 DR H.A.M, VAN DER VOSSEN graduated from Wageningen University in 1964 with an Ir (MSc) degree in tropical agronomy and plant breeding. He was re search officer-in-charge of the Oil Palm Research Centre at Kade, Ghana from 1964 to 1971 and head of the Coffee Breeding Unit at the Coffee Research Sta tion near Ruiru, Kenya, from 1971 to 1981. He obtained his PhD degree from Wageningen Agricultural University in 1974 with a thesis on breeding and quantitative genetics in the oil palm. In 1981 he joined Sluis & Groot Seed Company at Enkhuizen (Netherlands) as research manager and subsequently became director of the breeding programmes for vegetable and flower seed crops. After early retirement in 1993 he went overseas once more as seed in dustry adviser to the Ministry ofAgricultur e in Dhaka, Bangladesh until 1996. Since that time he has been active as a freelance consultant in plant breeding, and has undertaken assignments concerning vegetable seeds, cereal crops, co coa, oil palm and coffee. He has published over 40 scientific papers and written a chapter on breeding in two handbooks on coffee, contributed as co-author and associate editor of PROSEA No 8 Vegetables and as co-author and co-editor of PROSEA No 16 Stimulants. DR B.E. UMALI graduated from the University of the Philippines Los Banos (UPLB) in 1975 with a BSc degree in agriculture (major in horticulture). -

Community Baboon Sanctuary
Management Plan for the Community Baboon Sanctuary Linking Conservation and Sustainable Development 2013-2018 CBS Management Plan 2013-2018 Funding Provided by: Prepared by: Dr. Jon Lyon Department of Biology Merrimack College North Andover, MA 01845 USA 31 August 2013 2 CBS Management Plan 2013-2018 Executive Summary The Uniqueness of the Community Baboon Sanctuary The Community Baboon Sanctuary (CBS) was established in 1985 and is located on the northern coastal plain of Belize. It is geographically centered on the Village of Bermudian Landing in the lower Belize River Valley (Longitude 17o 55’ 65”N; Latitude: -88o 53’ 07” W). The CBS is an IUCN Category IV protected area but it is a Community Conserved Area where individual landowner participation is completely voluntary and based on a pledge system. The present membership is some 170 landholders and includes members of seven area villages, Flowers Bank, Scotland Half-Moon, Isabella Bank, St. Paul’s Bank, Willows Bank, Double Head Cabbage, and Bermudian Landing. The CBS covers some 14,800 acres. The Community Baboon Sanctuary (CBS) is unlike any other protected area or wildlife sanctuary in Belize. The nature and functioning of the CBS is completely embedded within the seven Belize River Valley communities that comprise it and it is inextricably linked to the Kriol culture and history of the region. The sanctuary nature of the CBS exists because of the long-held cultural appreciation for the black howler monkeys (Alouatta pigra) by the people that co-inhabit the region. Villagers in the Belize River Valley have very rarely ever hunted howlers for food and rarely engaged in capturing them for pets or the pet trade.