Preventing Wild Boar Sus Scrofa Damage – Considerations for Wild Boar Management in Highly Fragmented Agroecosystems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PDF Herunterladen
1 700.11 Verordnung zum Polizeigesetz Vom 9. Februar 1999 (Stand 1. April 2020) Der Regierungsrat des Kantons Basel-Landschaft, gestützt auf § 74 Abs. 2 der Verfassung des Kantons Basel-Landschaft vom 17. Mai 19841), * beschliesst: 1 Organisation der Polizei Basel-Landschaft § 1 * … § 2 * … § 3 * … § 4 * … § 5 Polizeiliche Grade * 1 Es bestehen folgende polizeiliche Grade, die sich aus den Funktionen erge- ben: * a. * Oberst, b. * Oberstleutnant, c. * Major, d. * Hauptmann, e. * Oberleutnant, f. * Leutnant, g. * Adjutant, h. * Feldweibel mit besonderen Aufgaben, i. * Feldweibel, j. * Wachtmeister mit besonderen Aufgaben, 1) SGS 100 * Änderungstabellen am Schluss des Erlasses GS 33.0605 2 700.11 k. * Wachtmeister, l. * Korporal, m. * Gefreiter, n. * Polizist, o. * Aspirant. 2 … * 3 … * 4 … * 5 … * § 5a * Sicherheitsassistenten und Sicherheitsassistentinnen 1 Sicherheitsassistenten und Sicherheitsassistentinnen erfüllen insbesondere folgende Aufgaben: * a. * Betreuung, Überwachung, Durchsuchung, Begleitung, Transport und Vor- führung von angehaltenen, festgenommenen und inhaftierten Personen; b. * Durchsetzung sitzungspolizeilicher Massnahmen bei Verhandlungen vor Gericht, Strafverfolgungsbehörden und anderen amtlichen Stellen, na- mentlich Zutrittskontrollen, Durchsuchungen und Wegweisungen; c. * Anhaltung und Zuführung von Personen im Auftrag von Behörden, ge- stützt auf deren Anordnungen; d. * Unterstützung von Behörden bei Amtshandlungen, bei Bedarf und aus- drücklicher Anordnung der zuständigen Behörde unter Anwendung unmit- telbaren -

4434 Hölstein, 22
Gemeindenachrichten Waldenburgertal Arboldswil, Bennwil, Hölstein, Lampenberg, Langenbruck, Liedertswil, Niederdorf, Oberdorf, Titterten und Waldenburg vom 25. Januar 2021 Vor-Registrierung für Impftermine ab Dienstag, 26. Januar möglich Der kantonale Krisenstab teilt mit: Zurzeit stehen nur sehr wenig Impfdosen zur Verfügung. Der Kanton Basel-Landschaft will dennoch dem Wunsch der Bevölkerung Rechnung tragen und bietet ab Dienstag, 26. Januar, eine Vor-Registrierung im Sinne einer Warteliste für Impftermine an. Diese Vor-Registrierung ist online via www.bl.ch/impfen oder telefonisch via Medgate-Infoline unter 058 387 77 07 möglich. Sobald neue Impfstoff-Lieferungen seitens Bund wieder garantiert und neue Impfter- mine vorhanden sind, werden diese dann an die eingetragenen Personen zugewiesen. Damit werden Impf- willige vom Druck entlastet, sich konstant über neue Impftermine informieren zu müssen. Zur Vor-Registrierung (Warteliste) sind aktuell Personen mit Wohnsitz im Kanton Basel-Landschaft zugelas- sen, welche eines der beiden nachfolgenden Kriterien erfüllen: - Alter über 75 Jahre (Geburtsdatum 30. Juni 1946 oder davor) - Personen mit chronischen Erkrankungen mit höchstem Risiko gemäss BAG-Definition (ärztlich un- terschriebenes Attest muss am Impftermin mitgebracht werden) Sirenentest am Mittwoch, 3. Februar Am Mittwoch, 3. Februar findet der jährliche schweizweite Sirenentest statt. Dabei wird die Funktionsbereit- schaft der rund 150 Sirenen im Kanton für den "Allgemeinen Alarm" und für den "Wasseralarm" getestet. Es sind keine Verhaltens- und Schutzmassnahmen zu ergreifen. Ausgelöst wird ab 13.30 Uhr das Zeichen “Allgemeiner Alarm“, ein regelmässig auf- und absteigender Heulton von einer Minute Dauer. Getestet wird nebst der zentralen Auslösung durch die Polizei Basel- Landschaft ab 13.45 Uhr eine zweite Alarmierung via Sirenen über eine separate Auslösestation. -

Mitteilungsblatt
MITTEILUNGSBLATT Gemeinde Bretzwil Offizielles Publikationsorgan der Gemeinde Bretzwil 31. Jahrgang Nr. 120 Erscheint vierteljährlich März 2016 Auflage: 370 Exemplare Redaktionsadresse: Gemeindeverwaltung Bretzwil, Kirchgasse 3, 4207 Bretzwil Redaktionsschluss: Jeweils der 10. des Monats vor Quartalsende Inserate: 1/1-Seite A4 Fr. 80.-- / ½-Seite A5 Fr. 40.-- / ¼-Seite A6 Fr. 20.-- / 1/8-Seite A7 Fr. 10.-- Öffnungszeiten der Gemeindeverwaltung: Montag, Mittwoch, Freitag 09.00 - 11.00 Uhr Donnerstag 17.00 - 19.00 Uhr Telefon 061 943 04 40 - Fax 061 943 04 41 - www.bretzwil.ch - [email protected] Sprechstunde des Gemeindepräsidenten nach Vereinbarung. Telefonische Anfragen Montag bis Freitag von 18.30 - 19.30 Uhr, 061 941 25 48. Für dringende Angelegenheiten jederzeit. 20 Jahre Guggenmusig Chuestallrugger Mitteilungsblatt der Gemeinde Bretzwil Nr. 120 Seite 2 Aus den Verhandlungen des Gemeinderates I ° SSSÖMMERUNGSBEITRAG 2015 SSSTIERENBERG Für den Stierenberg gilt ein Normalbesatz von 58.53 Normalstössen. Ein Normalstoss entspricht der Sömmerung einer Grossvieheinheit während 100 Tagen. Mit einem Besatz von 58.065 NST ist der vorgeschriebene Wert im Jahr 2015 praktisch punktgenau eingehalten worden. Gestützt auf die massgebenden Berechnungsgrundlagen ergab sich für die Bürgergemeinde Bretzwil bei einem aktuellen Ansatz von Fr. 400.-- pro Normalstoss ein Sömmerungsbeitrag von Fr. 23'412.--. Abgezogen von diesem Betrag wurde eine einmalige Busse von Fr. 3'000.-- für den im Jahr 2014 auf dem Stierenberg unerlaubt auf den Wiesen und Weiden ausgebrachten alpfremden Dünger. Die Höhe der Busse entspricht in etwa den jährlichen Einsparungen für den zukünftig nicht mehr erforderlichen Ankauf von Dünger, so dass dieser Vorgang finanziell gesehen keinen Einfluss auf die Rechnung 2015 der Bürgergemeinde hatte. -

Geschäftsbericht 2017 Spitex-Verband Baselland
Geschäftsbericht 2017 Spitex-Verband Baselland Verband Baselland Vorwort Geschäftsbericht 2017 «Wind of Change» lautet der Titel einer Rockballa- unterstützt und sich entschieden, neue Aufgaben de der «Scorpions», die – im September 1989 von zu übernehmen. Die Suche nach einer neuen Ge- Scorpions-Sänger Klaus Meine komponiert und schäftsführung, welche langfristig den Verband im getextet – zur Hymne für den politischen Wandel Wandel steuert und die Chancen, die sich eben aus jener Zeit wurde. diesem Wandel ergeben, zu nutzen weiss, musste Auch wir spüren heute den Wind des Wandels – gut überlegt sein. Der neu gebildete Vorstand hat allerdings im Gesundheitswesen. Dieser Wind sich rasch zu einer starken Gruppe entwickelt und weht verstärkt auch im Kanton Basel-Landschaft. konnte dazu strategische Überlegungen anstellen. Am 16.11.2017 stimmte der Landrat dem Altersbe- Dass eine geeignete Person nicht sofort zur Ver- treuungs- und Pflegegesetz (APG) zu. Die Vorberei- fügung steht, hat den Vorstand nicht dazu verlei- tung der Vernehmlassung hat uns bereits im Jahr tet, eine übereilte Wahl zu treffen. Qualität vor Ge- 2016 beschäftigt und forderte uns auch in 2017 schwindigkeit. Umso mehr freuen wir uns auf den noch. Wir sind überzeugt, dass die Umsetzung Start mit dem neuen Geschäftsführer, Urs Roth, grosse Auswirkungen auf die ambulante Pflege welcher am 1.7.2018 sein Amt antreten wird. haben wird, und sehen diesen Veränderungen op- Die Mitglieder des Vorstandes haben in der Zeit des timistisch entgegen. Die begonnene Zusammen- Wandels viel geleistet, so übernahm jeder zusätzli- arbeit mit anderen Leistungserbringenden hat uns che Aufgaben. Die Tatsache, dass unsere adminis- gezeigt, dass in dieser die Stärke der Zukunft liegt. -

Mitteilungen Aus Verwaltung Und Gemeinderat
GEMEINDE LAMPENBERG MITTEILUNGSBLATT NR.01/2020 HAUPTSTRASSE 40 09. JANUAR 2020 4432 LAMPENBERG Amtliches Publikationsorgan der Gemeinde Lampenberg Erscheint 1 – 2 Mal monatlich Eingabeschluss für Meldungen aus den Vereinen: Jeweils bis Ende Monat, Publikation erfolgt im darauffolgenden Monat. Öffnungszeiten der Gemeindeverwaltung: jeweils Donnerstag 17.30 – 20.00 Uhr Sprechstunde der Gemeindepräsidentin: Donnerstag,17.30 - 19.30 Uhr / 079 401 71 02 Kontakt: 061 951 25 00 / 079 361 50 72 (Christine Wagner) / [email protected] www.lampenberg.ch Mitteilungen aus Verwaltung und Gemeinderat Viele kleine Menschen, die an vielen kleinen Orten, viele kleine Dinge tun, können das Gesicht der Welt verändern. aus Afrika Liebe Einwohnerinnen und Einwohner von Lampenberg Mit diesen ermunternden Worten wünsche ich Ihnen ein wundervolles, lebensfreudiges, erfülltes und zufriedenes neues Jahr! Beste Gesundheit möge Ihnen den Alltag erleichtern und eine Prise Humor möge Sie stets begleiten, um die Herausforderungen des täglichen Lebens im 2020 in Gelassenheit anzunehmen. Die Weisheit aus Afrika ermutigt uns, als Einwohnerin und Einwohner von Lampenberg zu einer gelungenen Gemeinschaft bei zu tragen. Jeder noch so kleine Gedanke und jede noch so kleine Handlung ist wichtig und bewegt die Geschichte in Lampenberg. Gute Begegnungen im Alltag, ein schönes Gespräch, ein liebevolles Wort, ein herzlicher Gruss, ein aufrichtiges Dankeschön, ein verständnisvoller Blick und eine wohlwollende Tat. Besonders in einer Zeit, in welcher in lauten Tönen grosse Bewegungen gefordert werden, erscheint es mir sehr wichtig, dass wir uns unserer eigenen Kraft bewusst sind und das Kleine von ganzem Herzen tun. Es braucht viele „kleine“ aktive Menschen an einem kleinen Ort wie Lampenberg, die viele kleine Dinge tun, damit sie das Gesicht von Lampenberg sind und jenes der Welt verändern können. -
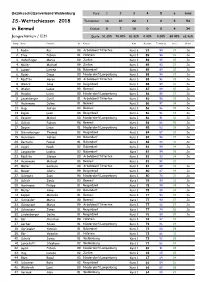
JS-Wettschiessen 2018 in Bennwil
Bezirksschützenverband Waldenburg Kurs 1 2 3 4 5 6 total JS-Wettschiessen 2018 Teilnehmer 16 10 22 1 0 5 54 in Bennwil Kränze 5 7 18 0 0 4 34 Jungschützen / U21 Quote 31.25% 70.00% 81.82% 0.00% 0.00% 80.00% 62.96% Rang Name Vorname JG Kursort Kurs Resultat Tiefschuss Serie Kranz 1. Rudin Kai 98 Arboldswil/Titterten Kurs 6 91 99 37 Ja 2. Frey Fabian 01 Hölstein Kurs 3 89 98 36 Ja 3. Häfelfinger Marco 99 Ziefen Kurs 3 89 95 35 Ja 4. Müller Michael 99 Ziefen Kurs 3 89 93 37 Ja 5. Lusser Lorenz 98 Bubendorf Kurs 6 89 85 32 Ja 6. Buser Diego 00 Niederdorf/Lampenberg Kurs 3 88 98 37 Ja 7. Räuftlin Aaron 99 Arboldswil/Titterten Kurs 3 88 96 35 Ja 8. Wehrli Silas 99 Reigoldswil Kurs 3 88 95 36 Ja 9. Wisler Lukas 99 Bennwil Kurs 3 87 89 35 Ja 10. Hajdini Lirim 00 Niederdorf/Lampenberg Kurs 3 86 99 37 Ja 11. Leuenberger Cyrill 01 Arboldswil/Titterten Kurs 3 86 98 35 Ja 12. Heinimann Celine 01 Bennwil Kurs 2 86 97 34 Ja 13. Hugi Adrian 00 Bennwil Kurs 3 86 96 35 Ja 14. Rigoni Leon 98 Reigoldswil Kurs 6 86 96 33 Ja 15. Beyeler Michel 00 Niederdorf/Lampenberg Kurs 3 86 91 36 Ja 16. Schick Fabian 98 Bennwil Kurs 6 86 86 35 Ja 17. Degen Linus 01 Niederdorf/Lampenberg Kurs 1 85 83 35 Ja 18. Dürrenberger Thomas 02 Reigoldswil Kurs 2 84 87 31 Ja 19. -

Vertrag Über Die Kindes- Und Erwachsenenschutzbehörde Und Die Berufsbeistandschaft Frenkentäler
l'1o,l 03 Vertrag über die Kindes- und Erwachsenenschutzbehörde und die Berufsbeistandschaft Frenkentäler vom 31. August 2012 Die Einwohnergemeinden Arboldswil, Bennwil, Bretzwil, Bubendorf, Hölstein, Lampenberg, Langenbruck, Lauwil, Liedertswil, Niederdorf, Oberdorf, Reigoldswil, Titterten, Waldenburg und Ziefen, gestützt auf § 60 Absatz 2 des Gesetzes vom 16. November 2006 über die Ein- führung des Zivilgesetzbuches (EG ZGB)1, vereinbaren: l. Allgemeine Bestimmungen § I Gemeinsame Kindes- und Erwachsenenschutzbehörde und Berufsbeistand. schaft Die Einwohnergemeinden Arboldswil, Bennwil, Bretzwil, Bubendorf, Hölstein, Lampenberg, Langenbruck, Lauwil, Liedertswil, Niederdorf, Oberdorf, Reigoldswil, Titterten, Waldenburg und Ziefen (kuz: Vertragsgemeinden) bestellen eine gemeinsame Kindes- und Erwachse- nenschutzbehörde (kuz: KESB) gemäss § 34bbi" Absatz 1 des Gemeindegesetzes2 und eine Berufsbeistandschaft. § 2 Ausführende Vereinbarung Die Gemeinderäte der Vertragsgemeinden regeln in einer separaten Vereinbarung ab- schliessend die Ausführungsbestimmungen zu diesem Vertrag. ll. Organisation § 3 Versammlung der Gemeindedelegierten 1 Die Gemeinderäte der Vertragsgemeinden entsenden aus ihrer Mitte je eine Person als Delegierte in die Versammlung der Gemeindedelegierten. 'sGSztt. 'sGS tao. Seite 'l von 9 2 Die Versammlung der Gemeindedelegierten nimmt folgende Aufgaben wahr: a. Festlegung derAnzahl Stellen der KESB und der Berufsbeistände b. Anstellung der Personen gemäss § 8 des Vertrages c. Genehmigung des Budgets, der lnvestitionen und der Rechnung der KESB und der Berufsbeistandschaft zuhanden der Vertragsgemeinden d. Erlass und Genehmigung der Geschäftsordnung der KESB und der Berufsbeistand- schaft 3 Die Versammlung der Gemeindedelegierten konstituiert sich selbst. Sie fasst ihre Be- schlüsse nach dem Mehrheitsprinzip. § 4 KESB und Berufsbeistandschaft 1 Der Sitz der KESB ist die Vertragsgemeinde, in welcher sich die Büroräumlichkeiten für sie und ihr Sekretariat befinden (Sitzgemeinde). 2 Die KESB umfasst: a. die Leitung b. -

Erneuerung Waldenburgerbahn Wasserbaukonzept Waldenburgertal - Vordere Frenke Und Vereinigte Frenke
W Erneuerung Waldenburgerbahn Wasserbaukonzept Waldenburgertal - Vordere Frenke und Vereinigte Frenke Konzept Technischer Bericht Version 1.2 l 16. November 2018 Projektverfasser: Bauherrschaft: Gruner Böhringer AG BLT Baselland Transport AG Michael Aggeler Antje Naujoks Reto Rotzler Fredi Schödler Titelbild: Auszug: www.Geoview.bl Darstellung von Gewässern (dunkelblau) und ÖV (Tram - grün, Bus - rot, Bahn - blau) Impressum Auftragsnummer 211'282'000 - 100 Auftraggeber BLT Baselland Transport AG, Grenzweg 1, 4104 Oberwil Datum 16. November 2018 Version 1.2 Autor(en) Antje Naujoks Gruner Böhringer AG Michael Aggeler Gruner Böhringer AG Freigabe --- Verteiler Reto Rotzler BLT Andreas Anetzeder Rapp Infra AG Jonas Woermann Tiefbauamt BL, GB Wasserbau Losingenieure Christian Bergerhoff Los 1 Gähler & Partner Markus Burgherr Los 2 INGE Frenketal (CES AG, Sutter AG) Bernhard Senn Los 3 Gruner AG Astrid Börner Los 4 IG Zukunft (Basler & Hofmann AG, Jauslin & Stebler) Bernhard Senn Los 5 Gruner AG Lukas Rentsch Los 6 IG Lampen-Stein (Aegerter & Bosshardt AG, CSD AG) Cédric Bachelard Los 7 Bachelard Wagner Architekten Datei K:\211282000_BLT_HWS_Waldenburgerbahn\Bericht\WKB_WBTal\WBK_WBtal_181116f.docx Seitenanzahl 16 Copyright Erneuerung Waldenburgerbahn Wasserbaukonzept Waldenburgertal - Vordere Frenke und Vereinigte Frenke Inhalt Änderungsverzeichnis iii Zusammenfassung iv 1 Projekt und Organisation 1 1.1 Bauherrschaft und Projektbeteiligte 1 1.2 Objektbeschreibung / Projektperimeter 1 2 Ausgangslage, Projektziele und Auftrag 2 2.1 Ausgangslage -

RVL Tarifzonenplan Mit TNW-Zonen 10 Und 40
nach Freiburg Notschrei nach nach Titisee Münstertal Muggen- Todtnauberg Feldberg brunn nach Freiburg Wiedener Eck 7 Fahl Belchen Branden- berg Todtnau Notschrei Multen Schlechtnau nach nach Titisee nach Münstertal Müllheim Utzenfeld Abzw.Präger Böden Todtnauberg Haldenhof 7 Geschwend Muggen- Feldberg brunn Neuenweg Präg Fachklinik nach nach nach Kandertal Freiburg Müllheim Müllheim Schönau Wiedener Eck Fahl 6 Bürchau 7 Wembach Schallsingen Marzell Belchen Branden- Mauchen 7 Hochkopfhaus Wies Raich berg Nieder- Todtnau eggenen Ehrsberg Multen Obereggenen 5 Schliengen 4 Sitzen- Schwand Schlechtnau Feuerbach kirch Liel Malsburg nach Mambach Abzw.Präger Adelsberg Müllheim Utzenfeld Hertingen Böden Bad Lehnacker Tegernau Atzenbach Haldenhof 7 Geschwend Bamlach Bellingen Riedlingen Kandern Sallneck Gresgen Zell im Gersbach Neuenweg Tannenkirch Wiesental Rheinweiler 5 6 Präg Welmlingen Schlächtenhaus Fachklinik RVL TarifzonenplanHolzen Hammerstein Wieslet nach nach nach Kandertal Enkenstein Hausen-Raitbach Freiburg Müllheim Müllheim Kleinkems Mappach Schönau Weitenau 6 6 Bürchau Wollbach Fahrnau Wembach Istein 4 Egringen Hägelberg Langenau Schallsingen Marzell Efringen- Wittlingen 7 Kirchen Mauchen Hochkopfhaus mit TNW-ZonenSchallbach 10 undSchlattholz 40 Raich 3 Hauingen Maulburg Hasel Wies Fischingen Rümmingen Nieder- Steinen Schopfheim Schopfheim Eimel- Richtung eggenen Ehrsberg West Wiechs dingen 1 Brombach/ Wehr Obereggenen 5 Binzen Hauingen Hüsingen Schliengen Märkt Tumringen Haagen/Messe 4 Sitzen- Schwand 3 Ötlingen Schwarzwaldstr. Feuerbach -

Horizontal Coordination, Democratic Legitimacy and Accountability
Paper prepared for ICPP Conference Milan, 1-4/7/2015 T02P07 - Comparing Horizontal Coordination of Policy Sectors Beyond a logic of effectiveness: Horizontal coordination, democratic legitimacy and accountability Eva Lieberherr Institute for Environmental Decisions Swiss Federal Institute of Technology (ETH) and Department of Environmental Social Sciences, Swiss Federal Institute of Aquatic Science and Technology Universitätstrasse 22 8092 Zurich, Switzerland +41 44 632 93 36 [email protected] Karin Ingold Institute of Political Science and Oeschger Centre for Climate Change Research, University of Berne and Department of Environmental Social Sciences, Swiss Federal Institute of Aquatic Science and Technology; [email protected] Abstract Horizontal coordination, where actors join together to accomplish a common task, has been applauded for its output legitimacy. However, such processes often face challenges due to opposition from local actors who raise concerns about democratic legitimacy and accountability. Moving beyond a logic of effectiveness, we aim to show how and why other forms of legitimacy such as input and throughput dimensions also affect horizontal coordination, in addition to output criteria. Beyond the assumed positive relationship between coordination and effectiveness, we additionally expect horizontal coordination to be (a) impeded by local actors’ fear of losing democratic legitimacy; and (b) fostered by accountability in terms of the steering capacity of the state. A comparative case study analysis of water supply structures at the regional level in Switzerland shows, in contrast to our expectation, that effectiveness has mixed impacts on horizontal coordination. Rather than being solely a positive factor for horizontal coordination, certain output criteria such as financial redistribution are found to be a key hindrance. -

Mietzinsgrenzwerte Baselland 2021
Mietzinsgrenzwerte Baselland 2021 (in den Gemeinden welche von Convalere betreut werden) Stand: 05.08.2021 2 3 4 5 junge Sozial- Gemeinde 1 Person NK Personen NK Personen NK Personen NK Personen NK 6 und mehr NK WG Zi. NK Erwachs. NK zimmer NK Aesch 900 135 1100 165 1300 195 1450 217.5 1600 240 auf Anfrage 550 82.5 Anwil 830 inkl. 110 inkl. 1300 inkl. 1400 inkl. 1500 inkl. auf Anfrage Arboldswil 850 inkl. 1200 inkl. 1450 inkl. 1750 inkl. 1900 inkl. auf Anfrage 500 inkl. 600 inkl. Arisdorf 1000 inkl. 1200 inkl. 1400 inkl. 1600 inkl. 1850 inkl. auf Anfrage 600 inkl. Augst 850 inkl. 1200 inkl. 1400 inkl. 1700 inkl. 1800 inkl. 1900 inkl. Bennwil 900 150 1000 200 1100 250 1200 250 1300 275 auf Anfrage 500 100 Buus 900 inkl. 1200 inkl. 1300 inkl. 1450 inkl. 1550 inkl. 1650 inkl. Duggingen 1000 1300 1600 1800 1800 2000 650 Ettingen 1000 1150 1300 1500 1700 1900 575 Frenkendorf 950 inkl. 1250 inkl. 1450 inkl. 1600 inkl. 1800 inkl. 1900 inkl. 650 inkl. Füllinsdorf 950 inkl. 1250 inkl. 1450 inkl. 1600 inkl. 1750 inkl. 1900 inkl. 625 inkl. Giebenach 900 inkl. 1100 inkl. 1500 inkl. 1700 inkl. 1700 inkl. 2100 inkl. Hölstein 910 inkl. 1100 inkl. 1470 inkl. 1620 inkl. 1800 inkl. auf Anfrage 550 inkl. 550 inkl. Lampenberg 800 1000 1200 1300 1400 1500 Laufen 750 150 920 180 1020 210 1250 250 1420 280 1670 330 550 inkl. Lauwil 850 inkl. 1200 inkl. 1450 inkl. 1750 inkl. 1900 inkl. auf Anfrage 500 inkl. -

Mitteilungsblatt Vom 21.09.2020
Gemeinde Oberdorf Nr. 198/20 E I N L A D U N G Z U R E I N W O H N E R G E M E I N D E V E R S A M M L U N G vom Montag, 21. September 2020, um 20.00 Uhr in der Mehrzweckhalle der Primarschule Oberdorf Traktanden: 1) Genehmigung Protokoll 2) Änderung Statuten Zweckverband der Musikschule beider Frenkentäler 3) Änderung Vertrag über den Schulrat der Musikschule beider Frenkentäler 4) Genehmigung Dienstbarkeitsvertrag unselbständiges Baurecht zugunsten der Jagdgesellschaft Oberdorf 5) Genehmigung Änderungen Wasserliefervertrag Auf Arten 6) Verschiedenes - Information Revision Zonenvorschriften Siedlung DER GEMEINDERAT Das Mitteilungsblatt mit den detaillierten Erläuterungen kann auf der Gemeindeverwal- tung einzeln oder als Abo bezogen werden. Ausserdem kann es auf unserer Home- page heruntergeladen werden: http://www.oberdorf.bl.ch / Politik / Gemeindeversammlung/ Sie erreichen uns unter: Tel. 061 965 90 90 oder [email protected] 2 Schutzkonzept Der Bundesrat hat aufgrund der Entwicklung der epidemiologischen Lage die Massnahmen zur Bekämpfung des neuen Coronavirus per 22.06.2020 weitgehend aufgehoben. Für alle öf- fentlich zugänglichen Einrichtungen und Betriebe sowie Veranstaltungen braucht es jedoch weiterhin ein Schutzkonzept. Die Vorgaben wurden indes vereinfacht und vereinheitlicht. Ab- stands- und Handhygieneregeln bleiben zentral und sollen helfen, Neuansteckungen und da- mit einen Wiederanstieg der Fallzahlen zu verhindern. Abstand Grundsätzlich ist der Mindestabstand zwischen zwei Personen von 1.5 Meter einzuhalten. Falls dieser nicht eingehalten werden kann, kann stattdessen das Tragen von Masken vorge- sehen werden. Händehygiene Die Händehygiene ist eine grundlegende Massnahme zur Verhinderung der Übertragung von Keimen. Allen Personen muss es möglich sein, sich regelmässig die Hände zu reinigen.