Detection of Some Elements in Sand (Reddish Orange and Black) By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role and Applications of Synchrotron Removal from Raman Spectra For
Vol. 1, No. 1, pp. 57-96, (October 2020) Aswan University Journal of Environmental Studies (AUJES) Online ISSN: 2735-4237, Print ISSN: 2735-4229 Journal homepage: http://aujes.aswu.edu.eg/ E-mail: [email protected] Original research Role and Applications of Synchrotron Removal from Raman Spectra for Quantitative Analysis of Cancer Tissues Alireza Heidari1,2* 1Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA 2American International Standards Institute, Irvine, CA 3800, USA Received: 28/8/2020 Accepted: 12/9/2020 © Unit of Environmental Studies and Development, Aswan University Abstract: In the current paper, the effect of presence and absence of synchrotron on quantitative analysis of sample is investigated using Fourier transform filters method. Using Raman spectroscopy on cancer tissues sample, which is one of the most important herbs, quantitative and qualitative analyses are performed. DNA/RNA of cancer cells was detected in the sample and the performance of Raman arrangement for measuring DNA/RNA of cancer cells concentration was evaluated at two parts using calibration graph. In the first part, spectra are containing synchrotron while in the second part, spectra are filtered and synchrotron are removed. Keywords: Quantitative Analysis, Cancer Tissues, Raman Spectroscopy, Calibration Graph, DNA/RNA, Synchrotron 1- INTRODUCTION Raman spectroscopy is a fast, cheap and inoffensive method for analyzing various types of solid, liquid and gas samples. One of the Raman spectroscopy problems about biological samples is presence of synchrotron in spectra. For removing synchrotron, there are various applied methods such as changing the laser wavelength or using Fourier transform arrangement and some theories such as shifted spectra and Fast Fourier Transform Filters [1–23]. -

Hadron Spectroscopy, Baryon Spectroscopy and Meson
Integrative Molecular Medicine Image ISSN: 2056-6360 Hadron spectroscopy, baryon spectroscopy and meson spectroscopy comparative study on malignant and benign human cancer cells and tissues under synchrotron radiation Alireza Heidari* Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA In the current study, we have experimentally and comparatively investigated and compared malignant human cancer cells and tissues before and after irradiating of synchrotron radiation using Hadron spectroscopy, Baryon spectroscopy and Meson spectroscopy. In the current study, we have experimentally and comparatively investigated and compared malignant human cancer cells and tissues before and after irradiating of synchrotron radiation using Hadron spectroscopy, Baryon spectroscopy and Meson spectroscopy. It is clear that malignant human cancer cells and tissues have gradually transformed to benign human cancer cells and tissues under synchrotron radiation with the passing of time (Figures 1-3) [1-198]. It can be concluded that malignant human cancer cells and tissues have gradually transformed to benign human cancer cells and tissues under synchrotron radiation with the passing of time (Figures 1-3) [1- 198]. Figure 2. Baryon spectroscopy analysis of malignant human cancer cells and tissues (a) before and (b) after irradiating of synchrotron radiation in transformation process to benign human cancer cells and tissues with the passing of time [1-198] *Correspondence to: Alireza Heidari, Faculty of Chemistry, California -

The Importance of Attenuated Total Reflectance Fourier Transform
ISSN: 2641-9475 ONCOGEN Research Article 2019; 2(2): 7 The Importance of Attenuated Total Reflectance Fourier Transform Infrared (ATR–FTIR) and Raman Biospectroscopy of Single–Walled Carbon Nanotubes (SWCNT) and Multi– Walled Carbon Nanotubes (MWCNT) in Interpreting Infrared and Raman Spectra of Human Cancer Cells, Tissues and Tu- mors Alireza Heidari*1,2, Jennifer Esposito1, Angela Caissutti1 1Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA 2American International Standards Institute, Irvine, CA 3800, USA *Corresponding author: Graphical Abstract Alireza Heidari In the current research, structure of Single–Walled Carbon American International Standards Institute, Nanotubes (SWCNT) and Multi–Walled Carbon Nanotubes Irvine, CA 3800, USA (MWCNT) was investigated by Attenuated Total Reflectance Fourier Transform Infrared (ATR–FTIR) and Raman spectrosco- Received : January 31, 2019 pies and it was combined with Carbon nanotubes to evaluate Published : March 12, 2019 its ability in act as radar absorber for interpreting infrared and Raman spectra of human cancer cells, tissues and tumors. In order to structurally characterize the sample and to determine characteristics related to degree of wave absorption by the sample, some analyses such as Back Scattering Raman, Atten- uated Total Reflectance Fourier Transform Infrared Biospec- troscopy (ATR–FTIR), X–Ray Diffraction (XRD) and Network An- alyzer (NA) were used. The structure of Single–Walled Carbon Nanotubes (SWCNT) and Multi–Walled Carbon Nanotubes (MWCNT) was clearly observable through active modes of Scanning Electron Microscope (SEM) image of Single–Walled Carbon Raman spectra and results of X–Ray Diffraction (XRD). Accord- Nanotubes (SWCNT) with 90000x zoom. ing to Network Analyzer (NA) spectrum analysis, the effect of nanotubes on wave absorption characteristics of sample was determined for interpreting infrared and Raman spectra of hu- man cancer cells, tissues and tumors. -

Detection of Some Elements...Pdf
Sudan University of Science and Technology College of Graduate Studies Detection of Some Elements in Kapo Powder milk and Nedo powder milk by Using X-Ray Fluorescence Device الكشف عن بعض العناصر في لبن البودرة كابو ولبن البودرة نيدو بإستخدام جهاز اﻷشعة السينية المتوهجة A dissertation Submitted as Partial Fulfillment of the Requirements for the Degree of Master of Science in Physics. By: Abeer Abdelrhman Ibrahim Supervisor: Dr. Nafeesa Badr Eldain December 2017 1 اﻵيـــــــــــة بسم الله الرحمن الرحيم قال الله تعالى: ﴿ ُق ْل َه ْل يَ ْس َت ِوي الَّ ِذي َن يَ ْعلَ ُمو َن َوالَّ ِذي َن ﻻ يَ ْعلَ ُمو َن ﴾ صدق الله العظيم ]الزمر:9[ I Dedication To the spirit of my pure father To my dear mother To my brothers and sisters To my husband To all my teachers To all my friends I dedicate you this research II Acknowledgements Firstly thanks to Allah for reconciling me Thanks to my beautiful family and my teachers and especially the supervisor of this research; Dr. Nafeesa Bar Eldain. III Abstract This study deals with the applications of spectroscopy, which is the detection of some elements of the Nido powder milk and Kapo powder milk and the concentration of these elements by X-Ray Fluorescence device and the comparison between them and to see the pH of the two samples, in milk Nido element chromium and its concentration 0.03%, manganese element, concentration 0.00%, Iron element and its concentration was 0.11%, nickel element, its concentration <0.001%, copper element, its concentration 0.00%, zinc element , its concentration 0.02 %and lead element and its concentration was 0.00%, and the PH of Nedo powder milk was 6.25. -

Enhancing the Raman Scattering for Diagnosis and Treatment of Human Cancer Cells, Tissues and Tumors Using Cadmium Oxide (Cdo) Nanoparticles
ISSN: 2572-4061 Heidari. J Toxicol Risk Assess 2018, 4:012 DOI: 10.23937/2572-4061.1510012 Volume 4 | Issue 1 Journal of Open Access Toxicology and Risk Assessment RESEARCH ARTICLE Enhancing the Raman Scattering for Diagnosis and Treatment of Hu- man Cancer Cells, Tissues and Tumors Using Cadmium Oxide (CdO) Nanoparticles Alireza Heidari* Check for Faculty of Chemistry, California South University, USA updates *Corresponding author: Alireza Heidari, Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA hancement Factor (EF) of Raman signal can reaches up Abstract to 1015 times. The main mechanism that affects EF of In the current paper, the Localized Surface Plasmon Res- onance (LSPR) effect induced by Cadmium Oxide (CdO) signal is electromagnetic mechanism and is induced by nanoparticles is used to observe Raman spectrum of hu- enhancing the scattered light by the Localized Surface man cancer cells, tissues and tumors. The diagnosis and Plasmon Resonance (LSPR) of metallic nanoparticles or treatment of human cancer cells, tissues and tumors in sam- in sharp points and other curvatures of Plasmon struc- ple is investigated through Nanomaterial Surface Energy tures. In this method, molecule should be placed at dis- Transfer (NSET) process from human cancer cells, tissues and tumors to the surface of nanoparticles, and Surface tance lower than 10 (nm) from the surface of nanopar- Enhanced Raman Scattering (SERS) process, as effective ticle [28-43]. factors for Raman spectrum detection. For interaction of hu- man cancer cells, tissues and tumors with Cadmium Oxide In recent years, a considerable attention has been (CdO) nanoparticles, colloidal state and Self-Assembled paid to pair and enhance the surface Plasmon fields in Monolayer (SAM) methods were used. -

Okadaic Acid Time-Resolved Absorption and Resonance FT-IR and Raman Biospectroscopy © 2019 Heidari A, Et Al
InternationalJournal of VIBGYOR Analytical and Bioanalytical Methods Okadaic Acid Time-Resolved Absorption and Resonance FT-IR and Raman Biospectroscopy and Density Functional Theory (DFT) Investigation of Vibronic-Mode Original Article: Open Access Coupling Structure in Vibrational Spectra Analysis Alireza Heidari1,2*, Jennifer Esposito1 and Angela Caissutti1 1Faculty of Chemistry, California South University, USA 2American International Standards Institute, USA Abstract Okadaic acid, C44H68O13, is a toxin produced by several species of dinoflagellates, and is known to accumulate in both marine sponges and shellfish. One of the primary causes of diarrhetic shellfish poisoning, Okadaic acid is a potent inhibitor of specific protein phosphatases and is known to have a variety of negative effects on cells. A polyketide, polyether derivative of a C38 fatty acid, Okadaic acid and other members of its family have shined light upon many biological processes both with respect to dinoflagellate polyketide synthesis as well as the role of protein phosphatases in cell growth. Parameters such as FT -IR and Raman vibrational wavelengths and intensities for single crystal Okadaic Acid are calculated using density functional theory and were compared with empirical results. The investigation about vibrational spectrum of cycle dimers in crystal with carboxyl groups from each molecule of acid was shown that it leads to create Hydrogen bonds for adjacent molecules. The current study aimed to investigate the possibility of simulating the empirical values. Analysis of vibrational spectrum of Okadaic Acid is performed based on theoretical simulation and FT-IR empirical spectrum and Raman empirical spectrum using density functional theory in levels of HF/6-31G*, HF/6-31++G**, MP2/6-31G, MP2/6-31++G**, BLYP/6-31G, BLYP/6-31++G**, B3LYP/6-31G and B3LYP6-31-HEG**. -

Time–Dependent Vibrational Spectral Analysis of Malignant and Benign Human Cancer Cells and Tissues Under Synchrotron Radiation
Open Access Journal of Cancer & Oncology Time–Dependent Vibrational Spectral Analysis of Malignant and Benign Human Cancer Cells and Tissues under Synchrotron Radiation Alireza H* Image Article Faculty of Chemistry, California South University, USA Volume 2 Issue 2 Received Date: April 18, 2018 *Corresponding Author: Alireza Heidari, Faculty of Chemistry, California South Published Date: May 17, 2018 University, 14731 Comet St. Irvine, CA 92604, USA, Email: [email protected] ; [email protected] Image Article In the current study, we have experimentally and Spectroscopy, Thermal Infrared Spectroscopy and Photo comparatively investigated and compared malignant thermal Infrared Spectroscopy. It is clear that malignant human cancer cells and tissues before and after human cancer cells and tissues have gradually irradiating of synchrotron radiation using Fourier transformed to benign human cancer cells and tissues Transform Infrared (FTIR) Spectroscopy, Attenuated under synchrotron radiation with the passage of time Total Reflectance Fourier Transform Infrared (ATR–FTIR) (Figures 1-15) [1-148]. It should be noted that time– Spectroscopy, Micro–Attenuated Total Reflectance dependent vibrational spectral analysis of malignant and Fourier Transform Infrared (Micro–ATR–FTIR) benign human cancer cells and tissues under synchrotron Spectroscopy, Macro–Attenuated Total Reflectance radiation are presented and illustrated in (Figures 1-15). Fourier Transform Infrared (Macro–ATR–FTIR) Furthermore, in the current study, we -

Ultrafast Processes in Molecules Visualized with Femtosecond Pump-Probe Photoelectron Spectroscopy
SCHRIFTENREIHE DES HZB · EXAMENSARBEITEN Ultrafast processes in molecules visualized with femtosecond pump-probe photoelectron spectroscopy Torsten Leitner Dissertation Institut für Methoden und Instrumentierung der Forschung mit Synchrotronstrahlung November 2012 HZB–B 37 Berichte des Helmholtz-Zentrums Berlin (HZB-Berichte) Das Helmholtz-Zentrum Berlin für Materialien und Energie gibt eine Serie von Berichten über Forschungs- und Entwicklungsergebnisse oder andere Aktivitäten des Zentrums heraus. Diese Berichte sind auf den Seiten des Zentrums elektronisch erhältlich. Alle Rechte an den Berichten liegen beim Zentrum außer das einfache Nutzungsrecht, das ein Bezieher mit dem Herunterladen erhält. Reports of the Helmholtz Centre Berlin (HZB-Berichte) The Helmholtz Centre Berlin for Materials and Energy publishes a series of reports on its research and development or other activities. The reports may be retrieved from the web pages of HZB and used solely for scientific, non-commercial purposes of the downloader. All other rights stay with HZB. ISSN 1868-5781 doi: http://dx.doi.org/10.5442/d0031 Helmholtz-Zentrum Berlin für Materialien und Energie · Hahn-Meitner-Platz 1 · D-14109 Berlin · Telefon +49 30 8062 0 · Telefax +49 30 8062 42181 · www.helmholtz-berlin.de Ultrafast processes in molecules visualized with femtosecond pump–probe photoelectron spectroscopy vorgelegt von Dipl.-Phys. Torsten Leitner aus Kirchham von der Fakult¨atII - Mathematik und Naturwissenschaften der Technischen Universit¨at Berlin zur Erlangung des akademischen Grades Doktor der Naturwissenschaften -Dr.rer.nat.- genehmigte Dissertation angefertigt am Helmholtz-Zentrum Berlin f¨urMaterialien und Energie Institut f¨urMethoden und Instrumentierung der Forschung mit Synchrotronstrahlung Promotionsausschuss: Vorsitzender: Prof. Dr. Mario D¨ahne Gutachter: Prof. Dr. Dr. -

Nuclear Resonant Inelastic X–Ray Scattering Spectroscopy (NRIXSS)
Global Imaging Insights Image ISSN: 2399-7397 Nuclear Resonant Inelastic X–Ray Scattering Spectroscopy (NRIXSS) and Nuclear Resonance Vibrational Spectroscopy (NRVS) Comparative Study on Malignant and Benign Human Cancer Cells and Tissues under Synchrotron Radiation Alireza Heidari* Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA In the current study, we have experimentally and comparatively investigated and compared malignant human cancer cells and tissues before and after irradiating of synchrotron radiation using Nuclear Resonant Inelastic X–Ray Scattering Spectroscopy (NRIXSS) and Nuclear Resonance Vibrational Spectroscopy (NRVS). It is clear that malignant human cancer cells and tissues have gradually transformed to benign human cancer cells and tissues under synchrotron radiation with the passing of time (Figures 1 and 2) [1-182]. It can be concluded that malignant human cancer cells and tissues have gradually transformed to benign human cancer cells and tissues under synchrotron radiation with the passing of time (Figures 1 and 2) [1-182]. Figure 2. Nuclear Resonance Vibrational Spectroscopy (NRVS) analysis of malignant human cancer cells and tissues (a) before and (b) after irradiating of synchrotron radiation in transformation process to benign human cancer cells and tissues with the passing of time [1-182] References (a) 1. Alireza Heidari, Christopher Brown (2015) Study of Composition and Morphology of Cadmium Oxide (CdO) Nanoparticles for Eliminating Cancer Cells. Journal of Nanomedicine Research, Volume 2, Issue 5, 20 Pages. 2. Alireza Heidari, Christopher Brown (2015) Study of Surface Morphological, Phytochemical and Structural Characteristics of Rhodium (III) Oxide (Rh2O3) Nanoparticles. International Journal of Pharmacology, Phytochemistry and Ethnomedicine, Volume 1, Pages 15–19. -

Using the Quantum Chemistry for Genesis of a Nano Biomembrane with a Combination of the Elements Be, Li, Se, Si, C and H
Journal of Nanomedicine Research Mini Review Open Access Using the quantum chemistry for genesis of a nano biomembrane with a combination of the elements Be, Li, Se, Si, C and H Abstract Volume 7 Issue 4 - 2018 Going beyond with imagination using quantum chemistry in calculations to obtain Ricardo Gobato,1 Alireza Heidari2 probable one new bio‒inorganic molecule, to the Genesis of a biomembrane with a 1Laboratory of Biophysics and Molecular Modeling Genesis, combination of the elements Be, Li, Se, Si, C and H. After calculation a bio‒inorganic State Secretariat for Education of Paraná, Brazil seed molecule from the previous combination, it led to the search for a molecule that 2Professor, Faculty of Chemistry, California South University, could carry the structure of a membrane. From a simple molecular dynamics, through USA classical calculations, the structure of the molecule was stabilized. An advanced study of quantum chemistry using ab initio, HF (Hartree‒Fock) method in various bases is Correspondence: Alireza Heidari, Professor, Faculty of applied and the expectation of the stabilization of the Genesis of this bio‒inorganic Chemistry, California South University, USA, was promising. The calculations made so far admit a seed molecule at this stage of the Email [email protected] quantum calculations of the arrangement of the elements we have chosen, obtaining a highly reactive molecule with the shape polar‒apolar‒polar. The molecule obtained Received: May 16, 2018 | Published: August 13, 2018 has a chemical structure C13H20BeLi2SeSi is plausible, correct and predicted by quantum chemistry through the RHF (Restricted Hartree‒Fock) method in the TZV (Triple Zeta Valence) sets bases. -

Saturated Spectroscopy and Unsaturated Spectroscopy, Respectively
Clinical Group Imaging Journal of Clinical and Medical Sciences DOI http://doi.org/10.17352/2455-8702.000036 ISSN: 2455-8702 DOI CC By Alireza Heidari* Clinical Image Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA Saturated Spectroscopy and Unsaturated Received: 11 January, 2018 Accepted: 12 February, 2018 Published: 15 February, 2018 Spectroscopy Comparative Study on *Corresponding author: Alireza Heidari, Faculty of Chemistry, California South University, 14731 Comet Malignant and Benign Human Cancer St. Irvine, CA 92604, USA, E-mail: Cells and Tissues with the Passage of https://www.peertechz.com Time under Synchrotron Radiation Image Article In the current study, we have experimentally and comparatively investigated and compared malignant human cancer cells and tissues before and after irradiating of synchrotron radiation using Saturated Spectroscopy and Unsaturated Spectroscopy, respectively. It is clear that malignant human cancer cells and tissues have gradually transformed to benign human cancer cells and tissues under synchrotron radiation with the passage of time (Figures 1,2) [1–163]. It should be noted that malignant human cancer cells and tissues were exposed under white synchrotron radiation for 30 days. Furthermore, there is a shift of the spectrum in Figure 2: Unsaturated Spectroscopy analysis of malignant human cancer all of spectra after irradiating of synchrotron radiation that cells and tissues. (a) before and (b) after irradiating of synchrotron radiation in transformation process to benign human cancer cells and tissues with the it is because of the malignant human cancer cells and tissues passage of time [1–163]. shrink post white synchrotron irradiation with the passage of time. -
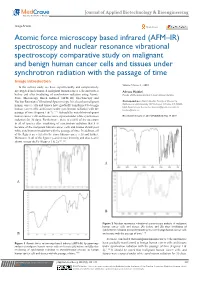
Atomic Force Microscopy Based Infrared (AFM–IR) Spectroscopy and Nuclear Resonance Vibrational Spectroscopy Comparative Study
Journal of Applied Biotechnology & Bioengineering Image Article Open Access Atomic force microscopy based infrared (AFM–IR) spectroscopy and nuclear resonance vibrational spectroscopy comparative study on malignant and benign human cancer cells and tissues under synchrotron radiation with the passage of time Image introduction Volume 5 Issue 3 - 2018 In the current study, we have experimentally and comparatively investigated and compared malignant human cancer cells and tissues Alireza Heidari before and after irradiating of synchrotron radiation using Atomic Faculty of Chemistry, California South University, USA Force Microscopy Based Infrared (AFM–IR) Spectroscopy and Nuclear Resonance Vibrational Spectroscopy. It is clear that malignant Correspondence: Alireza Heidari, Faculty of Chemistry, human cancer cells and tissues have gradually transformed to benign California South University, 14731 Comet St. Irvine, CA 92604, USA, Email [email protected], Alireza. human cancer cells and tissues under synchrotron radiation with the [email protected] passage of time (Figures 1 & 2).1–145 It should be noted that malignant human cancer cells and tissues were exposed under white synchrotron Received: February 21, 2018 | Published: May 14, 2018 radiation for 30 days. Furthermore, there is a shift of the spectrum in all of spectra after irradiating of synchrotron radiation that it is because of the malignant human cancer cells and tissues shrink post white synchrotron irradiation with the passage of time. In addition, all of