Two Thromboxane A2 Receptor Isoforms in Human Platelets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
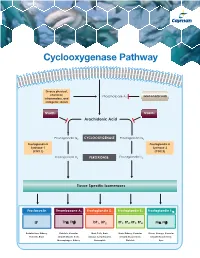
Cyclooxygenase Pathway
Cyclooxygenase Pathway Diverse physical, chemical, Phospholipase A Glucocorticoids inflammatory, and 2 mitogenic stimuli NSAIDs NSAIDs Arachidonic Acid Prostaglandin G2 CYCLOOXYGENASE Prostaglandin G2 Prostaglandin H Prostaglandin H Synthase-1 Synthase-2 (COX 1) (COX 2) Prostaglandin H2 PEROXIDASE Prostaglandin H2 Tissue Specific Isomerases Prostacyclin Thromboxane A2 Prostaglandin D2 Prostaglandin E2 Prostaglandin F2α IP TPα, TPβ DP1, DP2 EP1, EP2, EP3, EP4 FPα, FPβ Endothelium, Kidney, Platelets, Vascular Mast Cells, Brain, Brain, Kidney, Vascular Uterus, Airways, Vascular Platelets, Brain Smooth Muscle Cells, Airways, Lymphocytes, Smooth Muscle Cells, Smooth Muscle Cells, Macrophages, Kidney Eosinophils Platelets Eyes Prostacyclin Item No. Product Features Prostacyclin (Prostaglandin I2; PGI2) is formed from arachidonic acid primarily in the vascular endothelium and renal cortex by sequential 515211 6-keto • Sample Types: Culture Medium | Plasma Prostaglandin • Measure 6-keto PGF levels down to 6 pg/ml activities of COX and prostacyclin synthase. PGI2 is non-enzymatically 1α F ELISA Kit • Incubation : 18 hours | Development: 90-120 minutes | hydrated to 6-keto PGF1α (t½ = 2-3 minutes), and then quickly converted 1α Read: Colorimetric at 405-420 nm to the major metabolite, 2,3-dinor-6-keto PGF1α (t½= 30 minutes). Prostacyclin was once thought to be a circulating hormone that regulated • Assay 24 samples in triplicate or 36 samples in duplicate platelet-vasculature interactions, but the rate of secretion into circulation • NOTE: A portion of urinary 6-keto PGF1α is of renal origin coupled with the short half-life indicate that prostacyclin functions • NOTE : It has been found that normal plasma levels of 6-keto PGF may be low locally. -

Vasoactive Responses of U46619, PGF2 , Latanoprost, and Travoprost
Vasoactive Responses of U46619, PGF2␣, Latanoprost, and Travoprost in Isolated Porcine Ciliary Arteries Ineta Vysniauskiene, Reto Allemann, Josef Flammer, and Ivan O. Haefliger PURPOSE. To compare the vasoactive properties of the prosta- these responses can be modulated by SQ 29548 (TP-receptor noids U46619 (thromboxane A2 analogue), prostaglandin F2␣ antagonist) or AL-8810 (FP-receptor antagonist). (PGF2␣), latanoprost free acid, and travoprost free acid in isolated porcine ciliary arteries. METHODS. In a myograph system (isometric force measure- MATERIAL AND METHODS ment), quiescent vessels were exposed (cumulatively) to U46619, PGF , latanoprost, or travoprost (0.1 nM–0.1 mM). 2␣ Vessels Preparation Experiments were also conducted in the presence of SQ 29548 (TP-receptor antagonist; 3–10 M) or AL-8810 (FP-receptor Porcine eyes were obtained from an abattoir immediately after death. antagonist; 3–30 M). Contractions were expressed as the In cold modified Krebs-Ringer solution (NaCl 118 mM, KCl 4.7 mM, percentage of 100 mM potassium chloride-induced contrac- CaCl2 2.5 mM, K2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 25 mM, tions. glucose 11.1 mM, and EDTA 0.026 mM), ciliary arteries were dissected 5 RESULTS. In quiescent vessels, contractions (concentration–re- and cut into 2-mm segments. In an organ chamber, two 45- m Ϯ tungsten wires were passed through the vessel’s lumen and attached to sponse curves) induced by (0.1 mM) PGF2␣ (87.9% 3.5%), U46619 (66.7% Ϯ 4.1%), and latanoprost (62.9% Ϯ 3.6%) were a force transducer for isometric force measurements (Myo-Interface; JP more pronounced (P Յ 0.001) than those induced by tra- Trading, Aarhus, Denmark). -

Effect of Prostanoids on Human Platelet Function: an Overview
International Journal of Molecular Sciences Review Effect of Prostanoids on Human Platelet Function: An Overview Steffen Braune, Jan-Heiner Küpper and Friedrich Jung * Institute of Biotechnology, Molecular Cell Biology, Brandenburg University of Technology, 01968 Senftenberg, Germany; steff[email protected] (S.B.); [email protected] (J.-H.K.) * Correspondence: [email protected] Received: 23 October 2020; Accepted: 23 November 2020; Published: 27 November 2020 Abstract: Prostanoids are bioactive lipid mediators and take part in many physiological and pathophysiological processes in practically every organ, tissue and cell, including the vascular, renal, gastrointestinal and reproductive systems. In this review, we focus on their influence on platelets, which are key elements in thrombosis and hemostasis. The function of platelets is influenced by mediators in the blood and the vascular wall. Activated platelets aggregate and release bioactive substances, thereby activating further neighbored platelets, which finally can lead to the formation of thrombi. Prostanoids regulate the function of blood platelets by both activating or inhibiting and so are involved in hemostasis. Each prostanoid has a unique activity profile and, thus, a specific profile of action. This article reviews the effects of the following prostanoids: prostaglandin-D2 (PGD2), prostaglandin-E1, -E2 and E3 (PGE1, PGE2, PGE3), prostaglandin F2α (PGF2α), prostacyclin (PGI2) and thromboxane-A2 (TXA2) on platelet activation and aggregation via their respective receptors. Keywords: prostacyclin; thromboxane; prostaglandin; platelets 1. Introduction Hemostasis is a complex process that requires the interplay of multiple physiological pathways. Cellular and molecular mechanisms interact to stop bleedings of injured blood vessels or to seal denuded sub-endothelium with localized clot formation (Figure1). -

Activation of the Murine EP3 Receptor for PGE2 Inhibits Camp Production and Promotes Platelet Aggregation
Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation Jean-Etienne Fabre, … , Thomas M. Coffman, Beverly H. Koller J Clin Invest. 2001;107(5):603-610. https://doi.org/10.1172/JCI10881. Article The importance of arachidonic acid metabolites (termed eicosanoids), particularly those derived from the COX-1 and COX-2 pathways (termed prostanoids), in platelet homeostasis has long been recognized. Thromboxane is a potent agonist, whereas prostacyclin is an inhibitor of platelet aggregation. In contrast, the effect of prostaglandin E2 (PGE2) on platelet aggregation varies significantly depending on its concentration. Low concentrations of PGE2 enhance platelet aggregation, whereas high PGE2 levels inhibit aggregation. The mechanism for this dual action of PGE2 is not clear. This study shows that among the four PGE2 receptors (EP1–EP4), activation of EP3 is sufficient to mediate the proaggregatory actions of low PGE2 concentration. In contrast, the prostacyclin receptor (IP) mediates the inhibitory effect of higher PGE2 concentrations. Furthermore, the relative activation of these two receptors, EP3 and IP, regulates the intracellular level of cAMP and in this way conditions the response of the platelet to aggregating agents. Consistent with these findings, loss of the EP3 receptor in a model of venous inflammation protects against formation of intravascular clots. Our results suggest that local production of PGE2 during an inflammatory process can modulate ensuing platelet responses. Find the latest version: https://jci.me/10881/pdf Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation Jean-Etienne Fabre,1 MyTrang Nguyen,1 Krairek Athirakul,2 Kenneth Coggins,1 John D. -

Antiplatelet Effects of Prostacyclin Analogues: Which One to Choose in Case of Thrombosis Or Bleeding? Sylwester P
INTERVENTIONAL CARDIOLOGY Cardiology Journal 20XX, Vol. XX, No. X, XXX–XXX DOI: 10.5603/CJ.a2020.0164 Copyright © 20XX Via Medica REVIEW ARTICLE ISSN 1897–5593 eISSN 1898–018X Antiplatelet effects of prostacyclin analogues: Which one to choose in case of thrombosis or bleeding? Sylwester P. Rogula1*, Hubert M. Mutwil1*, Aleksandra Gąsecka1, Marcin Kurzyna2, Krzysztof J. Filipiak1 11st Chair and Department of Cardiology, Medical University of Warsaw, Poland 2Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Center of Postgraduate Education Medical, European Health Center Otwock, Poland Abstract Prostacyclin and analogues are successfully used in the treatment of pulmonary arterial hypertension (PAH) due to their vasodilatory effect on pulmonary arteries. Besides vasodilatory effect, prostacyclin analogues inhibit platelets, but their antiplatelet effect is not thoroughly established. The antiplatelet effect of prostacyclin analogues may be beneficial in case of increased risk of thromboembolic events, or undesirable in case of increased risk of bleeding. Since prostacyclin and analogues differ regarding their potency and form of administration, they might also inhibit platelets to a different extent. This review summarizes the recent evidence on the antiplatelet effects of prostacyclin and analogue in the treatment of PAH, this is important to consider when choosing the optimal treatment regimen in tailoring to an individual patients’ needs. (Cardiol J 20XX; XX, X: xx–xx) Key words: prostacyclin analogues, pulmonary arterial hypertension, platelets, antiplatelet effect, thrombosis, bleeding Introduction tiproliferative effects [4]. The main indication for PGI2 and analogues is advanced pulmonary arterial Since 1935 when prostaglandin was isolated hypertension (PAH) and peripheral vascular disor- for the first time [1], many scientists have focused ders [5]. -

Misoprostol Induces Relaxation of Human Corpus Cavernosum Smooth Muscle: Comparison to Prostaglandin E1
International Journal of Impotence Research (2000) 12, 107±110 ß 2000 Macmillan Publishers Ltd All rights reserved 0955-9930/00 $15.00 www.nature.com/ijir Misoprostol induces relaxation of human corpus cavernosum smooth muscle: comparison to prostaglandin E1 RB Moreland1, NN Kim1, A Nehra2, BG Parulkar3 and A Traish1,4* 1Department of Urology, Boston University School of Medicine, Boston, MA 02118, USA; 2Department of Urology, Mayo Clinic and Foundation, Rochester, MN 55905, USA; 3Department of Urology, University of Massachusetts Medical Center, Worcester, MA 01604, USA; and 4Department of Biochemistry, Boston University School of Medicine, Boston, MA 02118, USA Prostaglandin E1 (PGE1) relaxes trabecular smooth muscle by interacting with speci®c G-protein coupled receptors on human corpus cavernosum smooth muscle and increasing intracellular synthesis of cAMP. Misoprostol (CytotecTM), is an oral prostaglandin E analogue. The purpose of this study was to compare the functional activity of misoprostol with PGE1 in human corpus cavernosum and cultured human corpus cavernosum smooth muscle cells. Misoprostol, misoprostol free acid or PGE1 induced dose-dependent relaxations in strips of human corpus cavernosum. At concentrations greater than 1076 M, tissue recontraction was observed with all three agents. This was abrogated by pretreatment with the thromboxane A2 receptor antagonist SQ29,548. From these observations, we conclude that misoprostol is activated by human corpus cavernosum in situ and relaxes phenylephrine-precontrated tissue -

Prostacyclin Synthesis by COX-2 Endothelial Cells
Roles of Cyclooxygenase (COX)-1 and COX-2 in Prostanoid Production by Human Endothelial Cells: Selective Up-Regulation of Prostacyclin Synthesis by COX-2 This information is current as of October 2, 2021. Gillian E. Caughey, Leslie G. Cleland, Peter S. Penglis, Jennifer R. Gamble and Michael J. James J Immunol 2001; 167:2831-2838; ; doi: 10.4049/jimmunol.167.5.2831 http://www.jimmunol.org/content/167/5/2831 Downloaded from References This article cites 36 articles, 23 of which you can access for free at: http://www.jimmunol.org/content/167/5/2831.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on October 2, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2001 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Roles of Cyclooxygenase (COX)-1 and COX-2 in Prostanoid Production by Human Endothelial Cells: Selective Up-Regulation of Prostacyclin Synthesis by COX-21 Gillian E. Caughey,2* Leslie G. -

Increased Prostacyclin and Thromboxane A2 Biosynthesis in Atherosclerosis (Arachidonic Acid) JAWAHAR L
Proc. Nati. Acad. Sci. USA Vol. 85, pp. 4511-4515, June 1988 Medical Sciences Increased prostacyclin and thromboxane A2 biosynthesis in atherosclerosis (arachidonic acid) JAWAHAR L. MEHTA*t, DANIEL LAWSON*, PAULETTE MEHTA*, AND TOM SALDEENt *University of Florida, College of Medicine and the Veterans Administration Medical Center, Gainesville, FL 32610; and tUniversity of Uppsala, 751 21 Uppsala, Sweden Communicated by George K. Davis, March 2, 1988 ABSTRACT It has been proposed that atherosclerotic PGI2 metabolite released in the urine of patients with severe arteries produce less prostacyclin (PGI2) than nonatheroscle- atherosclerosis, and they demonstrated increased PGI2 bio- rotic arteries do, thereby predisposing arteries to vasospasm synthesis in these patients relative to normal subjects. and thrombosis in vivo. We reexamined this concept by Since many previous studies employed bioassay or only measuring spontaneous as well as arachidonate-induced PGI2 radioimmunoassay (RIA) for measurement of PGI2, the biosynthesis in aortic segments from nonatherosclerotic and present studies were designed to reexamine the concept of cholesterol-fed atherosclerotic New Zealand White rabbits. altered PGI2 generation in atherosclerosis. We examined the Thromboxane A2 (TXA2) generation was also measured. For- uptake and incorporation of labeled arachidonic acid into the mation of PGI2, as well as TXA2, as measured by radio- phospholipids ofthe vessel wall and subsequent arachidonate immunoassay (RIA) of their metabolites, was increased in release and conversion to major metabolites of and atherosclerotic aortic segments relative to nonatherosclerotic PGI2 segments (P S 0.05) at 0, 5, 10, 15, and 30 min of incubation TXA2 in both normal and atherosclerotic vessels. with arachidonate. Pretreatment of arterial segments with indomethacin inhibited PGI2 as well as TXA2 formation, MATERIALS AND METHODS whereas pretreatment with the selective TXA2 inhibitor OKY- 046 inhibited only TXA2 release, thus confirming the identity Induction of Atherosclerosis. -

Stable Derivatives of Thromboxane A2 with Differential Effects On
Proc. Natl. Acad. Sci. USA Vol. 86, pp. 5600-5604, July 1989 Medical Sciences Difluorothromboxane A2 and stereoisomers: Stable derivatives of thromboxane A2 with differential effects on platelets and blood vessels (receptors/contraction/aggregation/prostaglandins/10,10-difluorothromboxane A2 and analogues) THOMAS A. MORINELLI*, ANSELM K. OKWU*, DALE E. MAIS*, PERRY V. HALUSHKA*t, VARGHESE JOHNt, CHIEN-KUANG CHENt, AND JOSEF FRIEDt Departments of *Cell and Molecular Pharmacology and Experimental Therapeutics and of tMedicine, Medical University of South Carolina, Charleston, SC 29425; and tDepartment of Chemistry, The University of Chicago, Chicago, IL 60637 Contributed by JosefFried, April 13, 1989 ABSTRACT The present study reports on the selective this analogue is an antagonist (13). Other studies, also using effects on human platelets and canine saphenous veins of four different species, have shown differences in receptors in the stable difluorinated analogues and thromboxane A2 (TXA2), in two cell types (14, 15). Because different species were used which the characteristic 2,6-dioxa[3.1.1]bicycloheptane struc- as the source of platelets and blood vessels, these differences ture of TXA2 has been retained. The four compounds differ in may be species-selective rather than represent actual differ- their stereochemistry of the 5,6 double bond and/or the ences in the receptors. 15-hydroxyl group. Only 10,10-difluoro-TXA2 (compound I) Recent studies, however, have provided stronger evidence with the natural stereochemistry of TXA2 was an agonist in for different TXA2/PGH2 receptors in platelets and in blood both platelets and canine saphenous veins (EC50 = 36 ± 3.6 nM vessels (12, 16, 17). -

Long-Term Effects of Dietary Marine Omega-3 Fatty Acids Upon Plasma and Cellular Lipids, Platelet Function, and Eicosanoid Formation in Humans
Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. C von Schacky, … , S Fischer, P C Weber J Clin Invest. 1985;76(4):1626-1631. https://doi.org/10.1172/JCI112147. Research Article We studied the incorporation and metabolism of eicosapentanoic (EPA) and docosahexaenoic acid in six human volunteers who supplemented their normal Western diet for 5 mo daily with 10-40 ml of cod liver oil, rich in omega-3 polyunsaturated fatty acids. EPA and docosahexaenoic acid were incorporated into the total phospholipids of plasma, platelets, and erythrocytes in a dose- and time-dependent manner. During omega-3 fatty acid ingestion serum triacylglycerols were lowered and platelet aggregation upon low doses of collagen was reduced. Concomitantly, formation and excretion of prostanoids showed a characteristic change. As measured in serum from whole clotted blood, thromboxane A3 was formed in small amounts, whereas thromboxane A2 formation was reduced to 50% of control values. Excretion of the main urinary thromboxane A metabolites was unaltered in subjects with low basal excretion rates, but decreased markedly in two subjects with high control values. As determined from the main urinary metabolite, prostaglandin I3 was formed from EPA at rates up to 50% of unaltered prostaglandin I2 formation. The biochemical and functional changes observed lasted for the entire supplementation period of 5 mo and were reversible within 12 wk after cessation of cod liver oil intake. Favorable changes induced by long-chain omega-3 fatty acids include a dose-related and sustained shift of the prostaglandin I/thromboxane A balance to a more antiaggregatory and vasodilatory state. -

Antagonism of the Prostaglandin D Receptors DP and CRTH2 As An
REVIEWS Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases Roy Pettipher*, Trevor T. Hansel‡ and Richard Armer* Abstract | Immunological activation of mast cells is an important trigger in the cascade of inflammatory events leading to the manifestation of allergic diseases. Pharmacological studies using the recently discovered DP1 and CRTH2 antagonists combined with genetic analysis support the view that these receptors have a pivotal role in mediating aspects of allergic diseases that are resistant to current therapy. This Review focuses on the emerging roles that DP1 and CRTH2 (also known as DP2) have in acute and chronic aspects of allergic diseases and proposes that, rather than having opposing actions, these receptors have complementary roles in the initiation and maintenance of the allergy state. We also discuss recent progress in the discovery and development of selective antagonists of these receptors. Prostaglandin Prostaglandins D2 (PGD2) is an acidic lipid mediator that leads to the rapid production of PGD2, which can be Acidic lipids derived from the is derived from arachidonic acid by the sequential action detected in the bronchoalveolar lavage fluid within metabolism of arachidonic acid of cyclooxygenase(s) (COX) and PGD2 synthase(s). The minutes, reaching biologically active levels at least by the action of cyclo- COX(s) convert arachidonic acid in a two-step process 150-fold higher than pre-allergen levels10. Local anti- oxygenase enzymes and to first PGG and then PGH . These unstable endoper- gen challenge also stimulates PGD production in the downstream synthase 2 2 2 11 enzymes. Prostaglandins have oxide intermediates are converted to PGD2 by either the nasal mucosa of patients with allergic rhinitis and in (FIG. -

The Roles of Various Prostaglandins in Fibrosis: a Review
biomolecules Review The Roles of Various Prostaglandins in Fibrosis: A Review Ke Li, Jing Zhao, Mingxuan Wang, Lingzhi Niu, Yuanping Wang, Yanxia Li and Yajuan Zheng * Department of Ophthalmology, The Second Hospital of Jilin University, Changchun 130000, China; [email protected] (K.L.); [email protected] (J.Z.); [email protected] (M.W.); [email protected] (L.N.); [email protected] (Y.W.); [email protected] (Y.L.) * Correspondence: [email protected] Abstract: Organ fibrosis is a common pathological result of various chronic diseases with multiple causes. Fibrosis is characterized by the excessive deposition of extracellular matrix and eventually leads to the destruction of the tissue structure and impaired organ function. Prostaglandins are produced by arachidonic acid through cyclooxygenases and various prostaglandin-specific synthases. Prostaglandins bind to homologous receptors on adjacent tissue cells in an autocrine or paracrine manner and participate in the regulation of a series of physiological or pathological processes, including fibrosis. This review summarizes the properties, synthesis, and degradation of various prostaglandins, as well as the roles of these prostaglandins and their receptors in fibrosis in multiple models to reveal the clinical significance of prostaglandins and their receptors in the treatment of fibrosis. Keywords: fibrosis; myofibroblast; PGE2; PGD2; PGI2; PGF2α; TXA2 Citation: Li, K.; Zhao, J.; Wang, M.; 1. Introduction Niu, L.; Wang, Y.; Li, Y.; Zheng, Y. The Roles of Various Prostaglandins in Organ fibrosis is a common pathological result of chronic tissue damage caused by Fibrosis: A Review. Biomolecules 2021, various etiological factors. This condition is often defined as a degenerative process of 11, 789.