Prostaglandin Analog Effects on Cerebrospinal Fluid Reabsorption Via Nasal Mucosa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Product Insert (PDF)
Product Information U-46619 Item No. 16450 CAS Registry No.: 56985-40-1 Formal Name: 9,11-dideoxy-9α,11α-methanoepoxy-prosta- 5Z,13E-dien-1-oic acid Synonym: 9,11-dideoxy-9α,11α-methanoepoxy COOH Prostaglandin F2α MF: C21H34O4 O FW: 350.5 Purity: ≥98% OH Stability: ≥2 years at -20°C Supplied as: A solution in methyl acetate Laboratory Procedures For long term storage, we suggest that U-46619 be stored as supplied at -20°C. It will be stable for at least two years. U-46619 is supplied as a solution in methyl acetate. To change the solvent, simply evaporate the methyl acetate under a gentle stream of nitrogen and immediately add the solvent of choice. Solvents such as DMSO, ethanol, and dimethyl formamide purged with an inert gas can be used. The solubility of U-46619 in these solvents is aproximately 100 mg/ml. Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant since organic solvents may have physiological effects at low concentrations. If an organic solvent-free solution of U-46619 is needed, it can be prepared by evaporating the methyl acetate and directly dissolving the neat oil in aqueous buffers. The solubility of U-46619 in PBS (pH 7.2) is approximately 2 mg/ml. We do not recommend storing the aqueous solution for more than one day. 1 U-46619 is a stable analog of the endoperoxide prostaglandin H2, and a TP receptor agonist. -

And NF-Κb-Dependent Mechanism
www.nature.com/scientificreports OPEN Prostaglandin I2 Attenuates Prostaglandin E2-Stimulated Expression of Interferon γ in a Received: 17 September 2015 Accepted: 11 January 2016 β-Amyloid Protein- and NF-κB- Published: 12 February 2016 Dependent Mechanism Pu Wang*, Pei-Pei Guan*, Xin Yu, Li-Chao Zhang, Ya-Nan Su & Zhan-You Wang Cyclooxygenase-2 (COX-2) has been recently identified as being involved in the pathogenesis of Alzheimer’s disease (AD). However, the role of an important COX-2 metabolic product, prostaglandin (PG) I2, in AD development remains unknown. Using mouse-derived astrocytes as well as APP/ PS1 transgenic mice as model systems, we firstly elucidated the mechanisms of interferonγ (IFNγ) regulation by PGE2 and PGI2. Specifically, PGE2 accumulation in astrocytes activated the ERK1/2 and NF-κB signaling pathways by phosphorylation, which resulted in IFNγ expression. In contrast, the administration of PGI2 attenuated the effects of PGE2 on stimulating the production of IFNγ via inhibiting the translocation of NF-κB from the cytosol to the nucleus. Due to these observations, we further studied these prostaglandins and found that both PGE2 and PGI2 increased Aβ1–42 levels. In detail, PGE2 induced IFNγ expression in an Aβ1–42-dependent manner, whereas PGI2-induced Aβ1–42 production did not alleviate cells from IFNγ inhibition by PGI2 treatment. More importantly, our data also revealed that not only Aβ1–42 oligomer but also fibrillar have the ability to induce the expression of IFNγ via stimulation of NF-κB nuclear translocation in astrocytes of APP/PS1 mice. The production of IFNγ finally accelerated the deposition of βA 1–42 in β-amyloid plaques. -

Role of Proaggregatory and Antiaggregatory Prostaglandins in Hemostasis
Role of proaggregatory and antiaggregatory prostaglandins in hemostasis. Studies with combined thromboxane synthase inhibition and thromboxane receptor antagonism. P Gresele, … , G Pieters, J Vermylen J Clin Invest. 1987;80(5):1435-1445. https://doi.org/10.1172/JCI113223. Research Article Thromboxane synthase inhibition can lead to two opposing effects: accumulation of proaggregatory cyclic endoperoxides and increased formation of antiaggregatory PGI2 and PGD2. The elimination of the effects of the cyclic endoperoxides by an endoperoxide-thromboxane A2 receptor antagonist should enhance the inhibition of hemostasis by thromboxane synthase blockers. We have carried out a series of double-blind, placebo-controlled, crossover studies in healthy volunteers to check if this hypothesis may be operative in vivo in man. In a first study, in 10 healthy male volunteers, the combined administration of the thromboxane receptor antagonist BM 13.177 and the thromboxane synthase inhibitor dazoxiben gave stronger inhibition of platelet aggregation and prolonged the bleeding time more than either drug alone. In a second study, in 10 different healthy male volunteers, complete inhibition of cyclooxygenase with indomethacin reduced the prolongation of the bleeding time by the combination BM 13.177 plus dazoxiben. In a third study, in five volunteers, selective cumulative inhibition of platelet TXA2 synthesis by low-dose aspirin inhibited platelet aggregation and prolonged the bleeding time less than the combination BM 13.177 plus dazoxiben. In vitro, in -
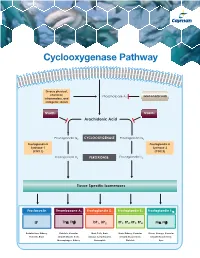
Cyclooxygenase Pathway
Cyclooxygenase Pathway Diverse physical, chemical, Phospholipase A Glucocorticoids inflammatory, and 2 mitogenic stimuli NSAIDs NSAIDs Arachidonic Acid Prostaglandin G2 CYCLOOXYGENASE Prostaglandin G2 Prostaglandin H Prostaglandin H Synthase-1 Synthase-2 (COX 1) (COX 2) Prostaglandin H2 PEROXIDASE Prostaglandin H2 Tissue Specific Isomerases Prostacyclin Thromboxane A2 Prostaglandin D2 Prostaglandin E2 Prostaglandin F2α IP TPα, TPβ DP1, DP2 EP1, EP2, EP3, EP4 FPα, FPβ Endothelium, Kidney, Platelets, Vascular Mast Cells, Brain, Brain, Kidney, Vascular Uterus, Airways, Vascular Platelets, Brain Smooth Muscle Cells, Airways, Lymphocytes, Smooth Muscle Cells, Smooth Muscle Cells, Macrophages, Kidney Eosinophils Platelets Eyes Prostacyclin Item No. Product Features Prostacyclin (Prostaglandin I2; PGI2) is formed from arachidonic acid primarily in the vascular endothelium and renal cortex by sequential 515211 6-keto • Sample Types: Culture Medium | Plasma Prostaglandin • Measure 6-keto PGF levels down to 6 pg/ml activities of COX and prostacyclin synthase. PGI2 is non-enzymatically 1α F ELISA Kit • Incubation : 18 hours | Development: 90-120 minutes | hydrated to 6-keto PGF1α (t½ = 2-3 minutes), and then quickly converted 1α Read: Colorimetric at 405-420 nm to the major metabolite, 2,3-dinor-6-keto PGF1α (t½= 30 minutes). Prostacyclin was once thought to be a circulating hormone that regulated • Assay 24 samples in triplicate or 36 samples in duplicate platelet-vasculature interactions, but the rate of secretion into circulation • NOTE: A portion of urinary 6-keto PGF1α is of renal origin coupled with the short half-life indicate that prostacyclin functions • NOTE : It has been found that normal plasma levels of 6-keto PGF may be low locally. -

Prostaglandin F2a Facilitates Hepatic Glucose Production Through Camkiig/P38/FOXO1 Signaling Pathway in Fasting and Obesity
1748 Diabetes Volume 67, September 2018 Prostaglandin F2a Facilitates Hepatic Glucose Production Through CaMKIIg/p38/FOXO1 Signaling Pathway in Fasting and Obesity Yuanyang Wang,1 Shuai Yan,2 Bing Xiao,2,3 Shengkai Zuo,2 Qianqian Zhang,2 Guilin Chen,1 Yu Yu,2,4 Di Chen,2,5 Qian Liu,1 Yi Liu,2 Yujun Shen,1 and Ying Yu1,2 Diabetes 2018;67:1748–1760 | https://doi.org/10.2337/db17-1521 Gluconeogenesis is drastically increased in patients Type 2 diabetes constitutes a major worldwide public health with type 2 diabetes and accounts for increased fasting burden and is expected to affect .642 million adults by plasma glucose concentrations. Circulating levels of 2040 (1). Type 2 diabetes is characterized by hyperglycemia, prostaglandin (PG) F2a are also markedly elevated in insulin resistance, and b-cell dysfunction, and often is diabetes; however, whether and how PGF2a regulates associated with low-grade chronic inflammation (2). Blood hepatic glucose metabolism remain unknown. Here, we glucose homeostasis is maintained by the balance between demonstrated that PGF2a receptor (F-prostanoid receptor hepatic glucose production (HGP) (glycogenolysis and glu- [FP]) was upregulated in the livers of mice upon fasting- coneogenesis) and glucose utilization by peripheral tissues. and diabetic stress. Hepatic deletion of the FP receptor In patients with type 2 diabetes, hepatic gluconeogenesis suppressed fasting-induced hepatic gluconeogenesis, is considerably elevated and contributes to both fasting whereas FP overexpression enhanced hepatic gluconeo- and postprandial hyperglycemia, and suppressing hepatic genesis in mice. FP activation promoted the expression gluconeogenesis improves insulin sensitivity and glucose METABOLISM of gluconeogenic enzymes (PEPCK and glucose-6- homeostasis, making it an attractive target for the treatment phosphatase) in hepatocytes in a FOXO1-dependent man- of diabetes (3). -

Vasoactive Responses of U46619, PGF2 , Latanoprost, and Travoprost
Vasoactive Responses of U46619, PGF2␣, Latanoprost, and Travoprost in Isolated Porcine Ciliary Arteries Ineta Vysniauskiene, Reto Allemann, Josef Flammer, and Ivan O. Haefliger PURPOSE. To compare the vasoactive properties of the prosta- these responses can be modulated by SQ 29548 (TP-receptor noids U46619 (thromboxane A2 analogue), prostaglandin F2␣ antagonist) or AL-8810 (FP-receptor antagonist). (PGF2␣), latanoprost free acid, and travoprost free acid in isolated porcine ciliary arteries. METHODS. In a myograph system (isometric force measure- MATERIAL AND METHODS ment), quiescent vessels were exposed (cumulatively) to U46619, PGF , latanoprost, or travoprost (0.1 nM–0.1 mM). 2␣ Vessels Preparation Experiments were also conducted in the presence of SQ 29548 (TP-receptor antagonist; 3–10 M) or AL-8810 (FP-receptor Porcine eyes were obtained from an abattoir immediately after death. antagonist; 3–30 M). Contractions were expressed as the In cold modified Krebs-Ringer solution (NaCl 118 mM, KCl 4.7 mM, percentage of 100 mM potassium chloride-induced contrac- CaCl2 2.5 mM, K2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 25 mM, tions. glucose 11.1 mM, and EDTA 0.026 mM), ciliary arteries were dissected 5 RESULTS. In quiescent vessels, contractions (concentration–re- and cut into 2-mm segments. In an organ chamber, two 45- m Ϯ tungsten wires were passed through the vessel’s lumen and attached to sponse curves) induced by (0.1 mM) PGF2␣ (87.9% 3.5%), U46619 (66.7% Ϯ 4.1%), and latanoprost (62.9% Ϯ 3.6%) were a force transducer for isometric force measurements (Myo-Interface; JP more pronounced (P Յ 0.001) than those induced by tra- Trading, Aarhus, Denmark). -

Effect of Prostanoids on Human Platelet Function: an Overview
International Journal of Molecular Sciences Review Effect of Prostanoids on Human Platelet Function: An Overview Steffen Braune, Jan-Heiner Küpper and Friedrich Jung * Institute of Biotechnology, Molecular Cell Biology, Brandenburg University of Technology, 01968 Senftenberg, Germany; steff[email protected] (S.B.); [email protected] (J.-H.K.) * Correspondence: [email protected] Received: 23 October 2020; Accepted: 23 November 2020; Published: 27 November 2020 Abstract: Prostanoids are bioactive lipid mediators and take part in many physiological and pathophysiological processes in practically every organ, tissue and cell, including the vascular, renal, gastrointestinal and reproductive systems. In this review, we focus on their influence on platelets, which are key elements in thrombosis and hemostasis. The function of platelets is influenced by mediators in the blood and the vascular wall. Activated platelets aggregate and release bioactive substances, thereby activating further neighbored platelets, which finally can lead to the formation of thrombi. Prostanoids regulate the function of blood platelets by both activating or inhibiting and so are involved in hemostasis. Each prostanoid has a unique activity profile and, thus, a specific profile of action. This article reviews the effects of the following prostanoids: prostaglandin-D2 (PGD2), prostaglandin-E1, -E2 and E3 (PGE1, PGE2, PGE3), prostaglandin F2α (PGF2α), prostacyclin (PGI2) and thromboxane-A2 (TXA2) on platelet activation and aggregation via their respective receptors. Keywords: prostacyclin; thromboxane; prostaglandin; platelets 1. Introduction Hemostasis is a complex process that requires the interplay of multiple physiological pathways. Cellular and molecular mechanisms interact to stop bleedings of injured blood vessels or to seal denuded sub-endothelium with localized clot formation (Figure1). -

Activation of the Murine EP3 Receptor for PGE2 Inhibits Camp Production and Promotes Platelet Aggregation
Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation Jean-Etienne Fabre, … , Thomas M. Coffman, Beverly H. Koller J Clin Invest. 2001;107(5):603-610. https://doi.org/10.1172/JCI10881. Article The importance of arachidonic acid metabolites (termed eicosanoids), particularly those derived from the COX-1 and COX-2 pathways (termed prostanoids), in platelet homeostasis has long been recognized. Thromboxane is a potent agonist, whereas prostacyclin is an inhibitor of platelet aggregation. In contrast, the effect of prostaglandin E2 (PGE2) on platelet aggregation varies significantly depending on its concentration. Low concentrations of PGE2 enhance platelet aggregation, whereas high PGE2 levels inhibit aggregation. The mechanism for this dual action of PGE2 is not clear. This study shows that among the four PGE2 receptors (EP1–EP4), activation of EP3 is sufficient to mediate the proaggregatory actions of low PGE2 concentration. In contrast, the prostacyclin receptor (IP) mediates the inhibitory effect of higher PGE2 concentrations. Furthermore, the relative activation of these two receptors, EP3 and IP, regulates the intracellular level of cAMP and in this way conditions the response of the platelet to aggregating agents. Consistent with these findings, loss of the EP3 receptor in a model of venous inflammation protects against formation of intravascular clots. Our results suggest that local production of PGE2 during an inflammatory process can modulate ensuing platelet responses. Find the latest version: https://jci.me/10881/pdf Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation Jean-Etienne Fabre,1 MyTrang Nguyen,1 Krairek Athirakul,2 Kenneth Coggins,1 John D. -

Prostaglandins and Other Lipid Mediators
Prostaglandins & other Lipid Mediators 122 (2016) 18–27 Contents lists available at ScienceDirect Prostaglandins and Other Lipid Mediators Original research article In silico modelling of prostacyclin and other lipid mediators to nuclear receptors reveal novel thyroid hormone receptor antagonist properties ∗ Noelia Perez Diaz, Mire Zloh, Pryank Patel, Louise S. Mackenzie Life and Medical Sciences, University of Hertfordshire, Hatfield AL10 9AB, UK a r t i c l e i n f o a b s t r a c t Article history: Prostacyclin (PGI2) is a key mediator involved in cardiovascular homeostasis, acting predominantly on Received 10 August 2015 two receptor types; cell surface IP receptor and cytosolic peroxisome proliferator activated receptor Received in revised form (PPAR) /␦. Having a very short half-life, direct methods to determine its long term effects on cells 19 November 2015 is difficult, and little is known of its interactions with nuclear receptors. Here we used computational Accepted 7 December 2015 chemistry methods to investigate the potential for PGI2, beraprost (IP receptor agonist), and GW0742 Available online 11 December 2015  ␦ (PPAR / agonist), to bind to nuclear receptors, confirmed with pharmacological methods. In silico screening predicted that PGI , beraprost, and GW0742 have the potential to bind to different Abbreviations: 2 nuclear receptors, in particular thyroid hormone  receptor (TR) and thyroid hormone ␣ receptor (TR␣). MLS, MLS000389544 Docking analysis predicts a binding profile to residues thought to have allosteric control on the TR ligand NSAID, non-steroidal anti-inflammatory drug binding site. Luciferase reporter assays confirmed that beraprost and GW0742 display TR and TR␣ −5 −6 × × PPAR/␦, peroxisome proliferator activated antagonistic properties; beraprost IC50 6.3 10 mol/L and GW0742 IC50 4.9 10 mol/L. -

Role of Curcumin on Tumor Angiogenesis in Hepatocellular Carcinoma
Naresuan University Journal 2008; 16(3): 239-254 239 Role of Curcumin on Tumor Angiogenesis in Hepatocellular Carcinoma Pornphrom Chintana Department of Physiology, Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand. Corresponding author. E-mail address: [email protected] (P. Chintana) Received 14 July 2008; accepted 24 December 2008 Summary Hepatocellular carcinoma (HCC) is a malignant tumor characterized by active neovascularization. Vascular endothelial growth factor (VEGF) is the most important angiogenic factor that regulates the HCC development. Overexpression of VEGF enhances the HCC tumor growth associated with increase of angiogenesis in the tumor, whereas suppression of VEGF attenuates the tumor growth. Similarly, the cyclooxygenase-2 (COX-2) expression stepwisely increases during hepatocarcinogenesis. Curcurmin has been shown to inhibit several angiogenic biomarkers including, VEGF and COX-2 expression. Therefore, curcumin could be used as a candidate for the combined drug treatment for HCC in the future. Keywords: Curcumin; Angiogenesis; Hepatocellular carcinoma; VEGF; COX-2 INTRODUCTION greater practical significance than non-selective cytotoxic therapies to control the tumor growth Hepatocellular carcinoma (HCC) is one of the and metastasis by targeting angiogenesis. Since, five most common cancers worldwide, with a many dietary and non-dietary phytochemicals do particularly high prevalence in Asian countries due not affect survival of normal cells and also possess to endemic hepatitis B virus infection (Parkin et al., anti-angiogenic as well as anti-tumorigenic 2001). In Thailand, it is the most common cause of activities, it could be a rationale approach to examine death in men (Vatanasapt et al., 2002). Surgery, their inhibitory effect on tumor angiogenesis. -

Antiplatelet Effects of Prostacyclin Analogues: Which One to Choose in Case of Thrombosis Or Bleeding? Sylwester P
INTERVENTIONAL CARDIOLOGY Cardiology Journal 20XX, Vol. XX, No. X, XXX–XXX DOI: 10.5603/CJ.a2020.0164 Copyright © 20XX Via Medica REVIEW ARTICLE ISSN 1897–5593 eISSN 1898–018X Antiplatelet effects of prostacyclin analogues: Which one to choose in case of thrombosis or bleeding? Sylwester P. Rogula1*, Hubert M. Mutwil1*, Aleksandra Gąsecka1, Marcin Kurzyna2, Krzysztof J. Filipiak1 11st Chair and Department of Cardiology, Medical University of Warsaw, Poland 2Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Center of Postgraduate Education Medical, European Health Center Otwock, Poland Abstract Prostacyclin and analogues are successfully used in the treatment of pulmonary arterial hypertension (PAH) due to their vasodilatory effect on pulmonary arteries. Besides vasodilatory effect, prostacyclin analogues inhibit platelets, but their antiplatelet effect is not thoroughly established. The antiplatelet effect of prostacyclin analogues may be beneficial in case of increased risk of thromboembolic events, or undesirable in case of increased risk of bleeding. Since prostacyclin and analogues differ regarding their potency and form of administration, they might also inhibit platelets to a different extent. This review summarizes the recent evidence on the antiplatelet effects of prostacyclin and analogue in the treatment of PAH, this is important to consider when choosing the optimal treatment regimen in tailoring to an individual patients’ needs. (Cardiol J 20XX; XX, X: xx–xx) Key words: prostacyclin analogues, pulmonary arterial hypertension, platelets, antiplatelet effect, thrombosis, bleeding Introduction tiproliferative effects [4]. The main indication for PGI2 and analogues is advanced pulmonary arterial Since 1935 when prostaglandin was isolated hypertension (PAH) and peripheral vascular disor- for the first time [1], many scientists have focused ders [5]. -

Prostaglandin D2 Inhibits Wound-Induced Hair Follicle Neogenesis Through the Receptor, Gpr44 Amanda M
ORIGINAL ARTICLE Prostaglandin D2 Inhibits Wound-Induced Hair Follicle Neogenesis through the Receptor, Gpr44 Amanda M. Nelson1,5, Dorothy E. Loy2,5, John A. Lawson3,4, Adiya S. Katseff1, Garret A. FitzGerald3,4 and Luis A. Garza1 Prostaglandins (PGs) are key inflammatory mediators involved in wound healing and regulating hair growth; however, their role in skin regeneration after injury is unknown. Using wound-induced hair follicle neogenesis (WIHN) as a marker of skin regeneration, we hypothesized that PGD2 decreases follicle neogenesis. PGE2 and PGD2 were elevated early and late, respectively, during wound healing. The levels of WIHN, lipocalin-type prostaglandin D2 synthase (Ptgds), and its product PGD2 each varied significantly among background strains of mice after wounding, and all correlated such that the highest Ptgds and PGD2 levels were associated with the lowest amount of regeneration. In addition, an alternatively spliced transcript variant of Ptgds missing exon 3 correlated with high regeneration in mice. Exogenous application of PGD2 decreased WIHN in wild-type mice, and PGD2 receptor Gpr44-null mice showed increased WIHN compared with strain-matched control mice. Furthermore, Gpr44-null mice were resistant to PGD2-induced inhibition of follicle neogenesis. In all, these findings demonstrate that PGD2 inhibits hair follicle regeneration through the Gpr44 receptor and imply that inhibition of PGD2 production or Gpr44 signaling will promote skin regeneration. Journal of Investigative Dermatology (2013) 133, 881–889; doi:10.1038/jid.2012.398; published online 29 November 2012 INTRODUCTION successfully transition through all phases of the hair cycle, Scar formation and tissue regeneration are opposite results of and include associated structures, such as sebaceous glands the wound healing process.