TOXINS Should “Bird-Proof” Their Homes to Provide a Safe and Enjoyable Environment for Their Companion Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moderate Levels of Glyphosate and Its Formulations Vary in Their Cytotoxicity
3 Biotech (2018) 8:438 https://doi.org/10.1007/s13205-018-1464-z ORIGINAL ARTICLE Moderate levels of glyphosate and its formulations vary in their cytotoxicity and genotoxicity in a whole blood model and in human cell lines with different estrogen receptor status L. K. S. De Almeida1 · B. I. Pletschke1 · C. L. Frost2 Received: 22 December 2017 / Accepted: 26 September 2018 © Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract In vitro studies were conducted to determine the short-term cytotoxic and genotoxic effects of pure glyphosate and two glyphosate formulations (Roundup ® and Wipeout ®) at concentrations relevant to human exposure using whole blood (cyto- toxicity) and various cancer cell lines (cytotoxicity and genotoxicity). Pure glyphosate (pure glyph) and Roundup® (Ro) showed similar non-monotonic toxicological profiles at low dose exposure (from 10 µg/ml), whereas Wipeout® (Wo) dem- onstrated a monotonic reduction in cell viability from a threshold concentration of 50 µg/ml, when tested in whole blood. We evaluated whether using various cancer cells (the estrogen-E2-responsive HEC1A, MCF7 and the estrogen-insensitive MDA-MB-231) exposed to moderate doses (75–500 µg/ml) would indicate varied toxicity and results indicated significant effects in the HEC1A cancer cells. A non-monotonic reduction in cell viability was observed in HEC1A exposed to pure glyph (75–500 µg/ml) and proliferative effects were observed after exposure to Wo (75, 125 and 250 µg/ml). Genotoxicity assessment (test concentration 500 µg/ml) demonstrated DNA damage in the HEC1A and MDA-MB-231 cells. Adjuvants and/or glyphosate impurities were potential contributing factors of toxicity based on the differential toxicities displayed by Ro and Wo in human whole blood and the HEC1A cells. -

Chelation Therapy
Medical Policy Chelation Therapy Table of Contents • Policy: Commercial • Coding Information • Information Pertaining to All Policies • Policy: Medicare • Description • References • Authorization Information • Policy History Policy Number: 122 BCBSA Reference Number: 8.01.02 NCD/LCD: N/A Related Policies None Policy Commercial Members: Managed Care (HMO and POS), PPO, and Indemnity Medicare HMO BlueSM and Medicare PPO BlueSM Members Chelation therapy in the treatment of the following conditions is MEDICALLY NECESSARY: • Extreme conditions of metal toxicity • Treatment of chronic iron overload due to blood transfusions (transfusional hemosiderosis) or due to nontransfusion-dependent thalassemia (NTDT) • Wilson's disease (hepatolenticular degeneration), or • Lead poisoning. Chelation therapy in the treatment of the following conditions is MEDICALLY NECESSARY if other modalities have failed: • Control of ventricular arrhythmias or heart block associated with digitalis toxicity • Emergency treatment of hypercalcemia. NaEDTA as chelation therapy is considered NOT MEDICALLY NECESSARY. Off-label applications of chelation therapy are considered INVESTIGATIONAL, including, but not limited to: • Alzheimer’s disease • Arthritis (includes rheumatoid arthritis) • Atherosclerosis, (e.g., coronary artery disease, secondary prevention in patients with myocardial infarction, or peripheral vascular disease) • Autism • Diabetes • Multiple sclerosis. 1 Prior Authorization Information Inpatient • For services described in this policy, precertification/preauthorization IS REQUIRED for all products if the procedure is performed inpatient. Outpatient • For services described in this policy, see below for products where prior authorization might be required if the procedure is performed outpatient. Outpatient Commercial Managed Care (HMO and POS) Prior authorization is not required. Commercial PPO and Indemnity Prior authorization is not required. Medicare HMO BlueSM Prior authorization is not required. -

Veterinary Toxicology
GINTARAS DAUNORAS VETERINARY TOXICOLOGY Lecture notes and classes works Study kit for LUHS Veterinary Faculty Foreign Students LSMU LEIDYBOS NAMAI, KAUNAS 2012 Lietuvos sveikatos moksl ų universitetas Veterinarijos akademija Neužkre čiam ųjų lig ų katedra Gintaras Daunoras VETERINARIN Ė TOKSIKOLOGIJA Paskait ų konspektai ir praktikos darb ų aprašai Mokomoji knyga LSMU Veterinarijos fakulteto užsienio studentams LSMU LEIDYBOS NAMAI, KAUNAS 2012 UDK Dau Apsvarstyta: LSMU VA Veterinarijos fakulteto Neužkre čiam ųjų lig ų katedros pos ėdyje, 2012 m. rugs ėjo 20 d., protokolo Nr. 01 LSMU VA Veterinarijos fakulteto tarybos pos ėdyje, 2012 m. rugs ėjo 28 d., protokolo Nr. 08 Recenzavo: doc. dr. Alius Pockevi čius LSMU VA Užkre čiam ųjų lig ų katedra dr. Aidas Grigonis LSMU VA Neužkre čiam ųjų lig ų katedra CONTENTS Introduction ……………………………………………………………………………………… 7 SECTION I. Lecture notes ………………………………………………………………………. 8 1. GENERAL VETERINARY TOXICOLOGY ……….……………………………………….. 8 1.1. Veterinary toxicology aims and tasks ……………………………………………………... 8 1.2. EC and Lithuanian legal documents for hazardous substances and pollution ……………. 11 1.3. Classification of poisons ……………………………………………………………………. 12 1.4. Chemicals classification and labelling ……………………………………………………… 14 2. Toxicokinetics ………………………………………………………………………...………. 15 2.2. Migration of substances through biological membranes …………………………………… 15 2.3. ADME notion ………………………………………………………………………………. 15 2.4. Possibilities of poisons entering into an animal body and methods of absorption ……… 16 2.5. Poison distribution -

Kingdom Class Family Scientific Name Common Name I Q a Records
Kingdom Class Family Scientific Name Common Name I Q A Records plants monocots Poaceae Paspalidium rarum C 2/2 plants monocots Poaceae Aristida latifolia feathertop wiregrass C 3/3 plants monocots Poaceae Aristida lazaridis C 1/1 plants monocots Poaceae Astrebla pectinata barley mitchell grass C 1/1 plants monocots Poaceae Cenchrus setigerus Y 1/1 plants monocots Poaceae Echinochloa colona awnless barnyard grass Y 2/2 plants monocots Poaceae Aristida polyclados C 1/1 plants monocots Poaceae Cymbopogon ambiguus lemon grass C 1/1 plants monocots Poaceae Digitaria ctenantha C 1/1 plants monocots Poaceae Enteropogon ramosus C 1/1 plants monocots Poaceae Enneapogon avenaceus C 1/1 plants monocots Poaceae Eragrostis tenellula delicate lovegrass C 2/2 plants monocots Poaceae Urochloa praetervisa C 1/1 plants monocots Poaceae Heteropogon contortus black speargrass C 1/1 plants monocots Poaceae Iseilema membranaceum small flinders grass C 1/1 plants monocots Poaceae Bothriochloa ewartiana desert bluegrass C 2/2 plants monocots Poaceae Brachyachne convergens common native couch C 2/2 plants monocots Poaceae Enneapogon lindleyanus C 3/3 plants monocots Poaceae Enneapogon polyphyllus leafy nineawn C 1/1 plants monocots Poaceae Sporobolus actinocladus katoora grass C 1/1 plants monocots Poaceae Cenchrus pennisetiformis Y 1/1 plants monocots Poaceae Sporobolus australasicus C 1/1 plants monocots Poaceae Eriachne pulchella subsp. dominii C 1/1 plants monocots Poaceae Dichanthium sericeum subsp. humilius C 1/1 plants monocots Poaceae Digitaria divaricatissima var. divaricatissima C 1/1 plants monocots Poaceae Eriachne mucronata forma (Alpha C.E.Hubbard 7882) C 1/1 plants monocots Poaceae Sehima nervosum C 1/1 plants monocots Poaceae Eulalia aurea silky browntop C 2/2 plants monocots Poaceae Chloris virgata feathertop rhodes grass Y 1/1 CODES I - Y indicates that the taxon is introduced to Queensland and has naturalised. -

Hearing in the Starling (Sturn Us Vulgaris): Absolute Thresholds and Critical Ratios
Bulletin of the Psychonomic Society 1986, 24 (6), 462-464 Hearing in the starling (Sturn us vulgaris): Absolute thresholds and critical ratios ROBERT J. DOOLING, KAZUO OKANOYA, and JANE DOWNING University of Maryland, College Park, Maryland and STEWART HULSE Johns Hopkins University, Baltimore, Maryland Operant conditioning and a psychophysical tracking procedure were used to measure auditory thresholds for pure tones in quiet and in noise for a European starling. The audibility curve for the starling is similar to the auditory sensitivity reported earlier for this species using a heart rate conditioning procedure. Masked auditory thresholds for the starling were measured at a number of test frequencies throughout the bird's hearing range. Critical ratios (signal-to-noise ratio at masked threshold) were calculated from these pure tone thresholds. Critical ratios in crease throughout the starling's hearing range at a rate of about 3 dB per octave. This pattern is similar to that observed for most other vertebrates. These results suggest that the starling shares a common mechanism of spectral analysis with many other vertebrates, including the human. Bird vocalizations are some of the most complex acous METHOD tic signals known to man. Partly for this reason, the Eu ropean starling is becoming a favorite subject for both SUbject The bird used in this experiment was a male starling obtained from behavioral and physiological investigations of the audi the laboratory of Stewart Hulse of 10hns Hopkins University. This bird tory processing of complex sounds (Hulse & Cynx, 1984a, had been used in previous experiments on complex sound perception 1984b; Leppelsack, 1978). -
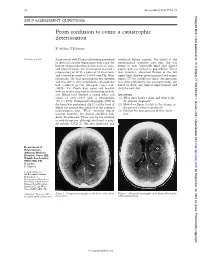
From Confusion to Coma: a Catastrophic Deterioration
52 Postgrad Med J 2001;77:52–55 Postgrad Med J: first published as 10.1136/pmj.77.903.53a on 1 January 2001. Downloaded from SELF ASSESSMENT QUESTIONS From confusion to coma: a catastrophic deterioration K Ashkan, F Johnston Answers on p 56. A previously well 45 year old woman presented ventilated before transfer. On arrival at the to the local casualty department with a one day neurosurgical intensive care unit, she was history of generalised headache, neck stiVness, found to have bilaterally fixed and dilated and blurred vision. On examination she had a pupils, with no corneal or gag reflexes. There temperature of 38°C, a pulse of 80 beats/min, was, however, abnormal flexion of the left and a blood pressure of 130/80 mm Hg. Neu- upper limb. She was given mannitol and urgent rologically, she had spontaneous eye opening repeat CT was carried out (fig 2). An operation and was able to obey commands, although she was then performed, but postoperatively she had confused speech (Glasgow coma scale failed to show any clinical improvement and (GCS), 14). Pupils were equal and reactive died the next day. with no cranial or peripheral neurological defi- cits. Blood tests showed a raised white cell Questions count of 20.9 × 109/l with a neutrophilia (1) What does figure 1 show and what is the (19.2 × 109/l). Computed tomography (CT) of diVerential diagnosis? the brain was performed (fig 1), on the basis of (2) How does figure 2 relate to the change in which the patient was referred to the regional the patient’s clinical condition? neurosurgical unit. -

A Pilot Study in Detoxification of Heavy Metals
The Role of Heavy Metal Detoxification in Heart Disease and Cancers : A Pilot Study in Detoxification of Heavy Metals Daniel Dugi, M.D. Sir Arnold Takemoto Presented at the WESCON Biomedicine and Bioengineering Conference Anaheim Convention Center The Future of Medicine Afternoon Session September 24, 2002 Anaheim, California USA 1 ABSTRACT Heavy metal detoxification has been shown to decrease cancer mortality by 90% in a 18-year controlled clinical study by Blumer and Cranton. Frustachi et. al. has shown a very strong correlation between cardiomyopathy (heart disease) and heavy metals accumulation in the coronary arteries and heart muscle. The role of heavy metal detoxification in the prevention and/or treatment of cancers and heart disease is paramount for optimum healing or prevention. Heavy metal accumulation can cause suppression of the immune system, bind receptor sites, inhibit proper enzyme systems, and lead to undesirable free-radical and oxidative functions. A pilot study utilizing a unique oral detoxifying concentrate, DeTox Max, containing true disodium EDTA, microencapsulated in essential phospholipids microspheres was utilized as a provocation, detoxifying agent for a 16 patient pilot study. Significant quantities of heavy metals were excreted in a 48-hour collection versus each patient’s baseline 24 hour collection. The pilot study results confirmed substantial excretion of heavy metals. A surprising outcome of the study was the remarkable clinical healing and significant increase in brain acuity in patients that occurred within 2 weeks after only one vial was utilized, compared to previous pre - provocation. 2 Detox MAX Clinical Study Proposal ¾ To assess the heavy metal detoxifying ability of Detox MAX, an oral detoxification agent containing 22 grams of essential phospholipids (EPL’s); micro- encapsulating 1 gram of sodium endetate. -

The Veterinarian's Guide
The Veterinarian’s Guide Bromethalin Addendum The Soft Bait Innovators™ Liphatech, Inc. • 3600 W. Elm Street • Milwaukee, WI 53209 • 1-888-331-7900 • www.liphatech.com NOTE: The information in this guide does not represent labeling and does not replace information on rodenticide labels relating to exposure of non-target species. Please read and follow all label directions on all rodenticide products you are using. Mentions of trade names in this publication does not imply endorsement of these products. Assault® is a registered trademark of PM Resources (St. Louis, MO) TakeDown™ and Cannon™ are trademarks of Liphatech, Inc. (Milwaukee, WI) Gunslinger® is a registered trademark of Liphatech, Inc. (Milwaukee, WI) Fastrac® is a registered trademark of Bell Labs (Madison, WI) Top Gun™ is a trademark of JT Eaton (Twinsburg, OH) Tomcat® and Rampage® are registered trademarks of Motomco (Madison, WI) Trounce® is a registered trademark of Agricultural Feeds (St. Louis, MO) At the time of this printing all information was deemed accurate and reliable. Liphatech, Inc. 3600 W. Elm Street Milwaukee, WI 53209 1-888-331-7900 www.liphatech.com October, 2016 The Veterinarian’s Guide - Bromethalin Addendum The Veterinarian’s Guide to Accidental Rodenticide Ingestion by Dogs and Cats focuses on anticoagulants. This addendum is intended to help veterinarians recognize and treat the symptoms of bromethalin toxicity in domestic animals. It describes bromethalin’s mode of action and how it is used as a rodenticide. In addition to reviewing the symptoms of bromethalin poisoning in domestic animals and the acute toxicity levels for dogs and cats (Table 5), we have also included the American Society for Prevention of Cruelty to Animals’ Animal Poison Control Center (ASPCA APCC) recommendations for decontamination (Tables 6 and 7). -

Parrots in the London Area a London Bird Atlas Supplement
Parrots in the London Area A London Bird Atlas Supplement Richard Arnold, Ian Woodward, Neil Smith 2 3 Abstract species have been recorded (EASIN http://alien.jrc. Senegal Parrot and Blue-fronted Amazon remain between 2006 and 2015 (LBR). There are several ec.europa.eu/SpeciesMapper ). The populations of more or less readily available to buy from breeders, potential factors which may combine to explain the Parrots are widely introduced outside their native these birds are very often associated with towns while the smaller species can easily be bought in a lack of correlation. These may include (i) varying range, with non-native populations of several and cities (Lever, 2005; Butler, 2005). In Britain, pet shop. inclination or ability (identification skills) to report species occurring in Europe, including the UK. As there is just one parrot species, the Ring-necked (or Although deliberate release and further import of particular species by both communities; (ii) varying well as the well-established population of Ring- Rose-ringed) parakeet Psittacula krameri, which wild birds are both illegal, the captive populations lengths of time that different species survive after necked Parakeet (Psittacula krameri), five or six is listed by the British Ornithologists’ Union (BOU) remain a potential source for feral populations. escaping/being released; (iii) the ease of re-capture; other species have bred in Britain and one of these, as a self-sustaining introduced species (Category Escapes or releases of several species are clearly a (iv) the low likelihood that deliberate releases will the Monk Parakeet, (Myiopsitta monachus) can form C). The other five or six¹ species which have bred regular event. -

Hypervitaminosis - an Emerging Pathological Condition
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Review Article Hypervitaminosis - An Emerging Pathological Condition J K Roop PG Department of Zoology, JC DAV College (Affiliated to Panjab University, Chandigarh), Dasuya-144205, District Hoshiarpur, Punjab, India ABSTRACT Vitamins are essential organic compounds that are required in small amounts to regulate various metabolic activities in the body. Prolonged and overconsumption of pharmaceutical forms of both water-soluble and fat-soluble vitamins may lead to toxicity and/or hypervitaminosis. Hypervitaminosis is an acute emerging pathological condition of the body due to excess accumulation of any of the vitamins. In case of acute poisoning with vitamin supplements/drugs, emergency assistance is required to detoxify the effects and restore the organization, structure and function of body’s tissues and organs. Sometimes death may occur due to intoxication to liver, kidney and heart. So, to manage any type of hypervitaminosis, proper diagnosis is essential to initiate eliminating the cause of its occurrence and accelerate the elimination of the supplement from the body. The present review discusses the symptoms of hypervitaminosis that seems to be a matter of concern today and management strategies to overcome toxicity or hypervitaminosis. Keywords: Hypervitaminosis, Toxicity, Vitamins, Vitamin pathology, Fat-soluble vitamin, Water- soluble vitamin. INTRODUCTION body, particularly in the liver. Vitamin B Vitamins are potent organic Complex and vitamin C are water- soluble. compounds present in small concentrations They are dissolved easily in food during in various fruits and vegetables. They cooking and a portion of these vitamins may regulate physiological functions and help in be destroyed by heating. -

Chelation Therapy
Corporate Medical Policy Chelation Therapy File Name: chelation_therapy Origination: 12/1995 Last CAP Review: 2/2021 Next CAP Review: 2/2022 Last Review: 2/2021 Description of Procedure or Service Chelation therapy is an established treatment for the removal of metal toxins by converting them to a chemically inert form that can be excreted in the urine. Chelation therapy comprises intravenous or oral administration of chelating agents that remove metal ions such as lead, aluminum, mercury, arsenic, zinc, iron, copper, and calcium from the body. Specific chelating agents are used for particular heavy metal toxicities. For example, desferroxamine (not Food and Drug Administration [FDA] approved) is used for patients with iron toxicity, and calcium-ethylenediaminetetraacetic acid (EDTA) is used for patients with lead poisoning. Note that disodium-EDTA is not recommended for acute lead poisoning due to the increased risk of death from hypocalcemia. Another class of chelating agents, called metal protein attenuating compounds (MPACs), is under investigation for the treatment of Alzheimer’s disease, which is associated with the disequilibrium of cerebral metals. Unlike traditional systemic chelators that bind and remove metals from tissues systemically, MPACs have subtle effects on metal homeostasis and abnormal metal interactions. In animal models of Alzheimer’s disease, they promote the solubilization and clearance of β-amyloid protein by binding to its metal-ion complex and also inhibit redox reactions that generate neurotoxic free radicals. MPACs therefore interrupt two putative pathogenic processes of Alzheimer’s disease. However, no MPACs have received FDA approval for treating Alzheimer’s disease. Chelation therapy has also been investigated as a treatment for other indications including atherosclerosis and autism spectrum disorder. -

AMADOR BIRD TRACKS Monthly Newsletter of the Amador Bird Club June 2014 the Amador Bird Club Is a Group of People Who Share an Interest in Birds and Is Open to All
AMADOR BIRD TRACKS Monthly Newsletter of the Amador Bird Club June 2014 The Amador Bird Club is a group of people who share an interest in birds and is open to all. Happy Father’s Day! Pictured left is "Pale Male" with offspring, in residence at his 927 Fifth Avenue NYC apartment building nest, overlooking Central Park. Perhaps the most famous of all avian fathers, Pale Male’s story 3 (continued below) ... “home of the rare Amadorian Combo Parrot” Dates of bird club President’s Message The Amador Bird Club meeting will meetings this year: be held on: • June 13* Hi Everyone, hope you are enjoying the Friday, June 13, 2014 at 7:30 PM • July 11 warmer weather as I know that some of the • August 8 birds are "getting into the swing of things" and Place : Administration Building, • Sept. 12** producing for all of you breeders. This month Amador County Fairgrounds, • October 10 we will be having a very interesting and Plymouth informative presentation on breeding and • November 14 showing English budgies by Mary Ann Silva. I • December 12 Activity : Breeding and Showing am hoping that you all can attend as it will be English Budgies by Mary Ann (Xmas Party) your loss if you miss this night. Silva *Friday-the-13 th : drive carefully! We will be signing up for the Fair booth Refreshments: Persons with ** Semi-Annual attendance and manning so PLEASE mark it last names beginning with S-Z. Raffle on your calendar. The more volunteers the easier it is for EVERYONE. Set-up is always the Wed.