Chelation Therapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Calcium Chelation on Digitalis-Induced Cardiac Arrhythmias by Paul Szekely and N
Br Heart J: first published as 10.1136/hrt.25.5.589 on 1 September 1963. Downloaded from Brit. Heart J., 1963, 25, 589. EFFECTS OF CALCIUM CHELATION ON DIGITALIS-INDUCED CARDIAC ARRHYTHMIAS BY PAUL SZEKELY AND N. A. WYNNE From the Cardiovascular Department, Newcastle General Hospital, and the Department ofPhysiology, King's College, University of Durham Received November 19, 1962 Several studies have already shown that cardiac arrhythmias caused by digitalis can be abolished by the induction of hypocalcaemia (Page and Real, 1955; Smith and Grinnell, 1955; Gubner and Kallman, 1957; Kabakow and Brothers, 1958; Surawicz et al., 1959; Cohen et al., 1959; Rosenbaum, Mason, and Seven, 1960; Surawicz, 1960; Soffer, Toribara, and Sayman, 1961). The rationale of inducing hypocalcemia as an anti-arrhythmic measure is based on experimental and clinical obser- vations relating to the direct myocardial action of calcium and to the interaction between calcium, potassium, and digitalis. Low calcium concentration decreases myocardial irritability (Brooks et al., 1955) and it also increases the intracellular potassium concentration (Rosenbaum et al., 1960). There is also a synergistic action between calcium and digitalis, which was observed in the presence of digitalis intoxication (Nalbandian et al., 1957). The present study was undertaken copyright. in order to assess the value of induced hypocalcemia in the management of cardiac arrhythmias caused by digitalis. MATERIAL AND METHODS Forty-eight experiments were carried out under general aneesthesia on 46 cats and 2 dogs. Cardiac arrhythmia was induced by the intravenous administration of tincture of digitalis as previously described http://heart.bmj.com/ (Szekely and Wynne, 1951). -

New Brunswick Drug Plans Formulary
New Brunswick Drug Plans Formulary August 2019 Administered by Medavie Blue Cross on Behalf of the Government of New Brunswick TABLE OF CONTENTS Page Introduction.............................................................................................................................................I New Brunswick Drug Plans....................................................................................................................II Exclusions............................................................................................................................................IV Legend..................................................................................................................................................V Anatomical Therapeutic Chemical (ATC) Classification of Drugs A Alimentary Tract and Metabolism 1 B Blood and Blood Forming Organs 23 C Cardiovascular System 31 D Dermatologicals 81 G Genito Urinary System and Sex Hormones 89 H Systemic Hormonal Preparations excluding Sex Hormones 100 J Antiinfectives for Systemic Use 107 L Antineoplastic and Immunomodulating Agents 129 M Musculo-Skeletal System 147 N Nervous System 156 P Antiparasitic Products, Insecticides and Repellants 223 R Respiratory System 225 S Sensory Organs 234 V Various 240 Appendices I-A Abbreviations of Dosage forms.....................................................................A - 1 I-B Abbreviations of Routes................................................................................A - 4 I-C Abbreviations of Units...................................................................................A -

A Pilot Study in Detoxification of Heavy Metals
The Role of Heavy Metal Detoxification in Heart Disease and Cancers : A Pilot Study in Detoxification of Heavy Metals Daniel Dugi, M.D. Sir Arnold Takemoto Presented at the WESCON Biomedicine and Bioengineering Conference Anaheim Convention Center The Future of Medicine Afternoon Session September 24, 2002 Anaheim, California USA 1 ABSTRACT Heavy metal detoxification has been shown to decrease cancer mortality by 90% in a 18-year controlled clinical study by Blumer and Cranton. Frustachi et. al. has shown a very strong correlation between cardiomyopathy (heart disease) and heavy metals accumulation in the coronary arteries and heart muscle. The role of heavy metal detoxification in the prevention and/or treatment of cancers and heart disease is paramount for optimum healing or prevention. Heavy metal accumulation can cause suppression of the immune system, bind receptor sites, inhibit proper enzyme systems, and lead to undesirable free-radical and oxidative functions. A pilot study utilizing a unique oral detoxifying concentrate, DeTox Max, containing true disodium EDTA, microencapsulated in essential phospholipids microspheres was utilized as a provocation, detoxifying agent for a 16 patient pilot study. Significant quantities of heavy metals were excreted in a 48-hour collection versus each patient’s baseline 24 hour collection. The pilot study results confirmed substantial excretion of heavy metals. A surprising outcome of the study was the remarkable clinical healing and significant increase in brain acuity in patients that occurred within 2 weeks after only one vial was utilized, compared to previous pre - provocation. 2 Detox MAX Clinical Study Proposal ¾ To assess the heavy metal detoxifying ability of Detox MAX, an oral detoxification agent containing 22 grams of essential phospholipids (EPL’s); micro- encapsulating 1 gram of sodium endetate. -

Post-Injury Calcium Chelation Rescues Skeletal Muscle Regeneration in Mice Matthew .D Magda University of Connecticut - Storrs, [email protected]
University of Connecticut OpenCommons@UConn Honors Scholar Theses Honors Scholar Program Summer 8-1-2013 Post-Injury Calcium Chelation Rescues Skeletal Muscle Regeneration in Mice Matthew .D Magda University of Connecticut - Storrs, [email protected] Follow this and additional works at: https://opencommons.uconn.edu/srhonors_theses Part of the Cell Biology Commons, and the Molecular Biology Commons Recommended Citation Magda, Matthew D., "Post-Injury Calcium Chelation Rescues Skeletal Muscle Regeneration in Mice" (2013). Honors Scholar Theses. 321. https://opencommons.uconn.edu/srhonors_theses/321 1 Post-Injury Calcium Chelation Rescues Skeletal Muscle Regeneration in Mice Honors Thesis Matthew Magda Research Advisor: Dr. Morgan Carlson Honors Advisor: Dr. Kenneth Noll August 2013 2 Abstract: Antibiotics, surgery and organ transplants have pushed average lifespans towards the upper limits of the human body. Drastically reduced morbidity from infection, toxins and traumatic injury have allowed ever greater portions of the populace can reach eighty or ninety years old before dying of old age. Despite the increased role of aging as a source of morbidity, many aspects of aging are poorly characterized. Sarcopenia, progressive muscle loss, and loss of adult myogenic potential, the ability to produce new muscle tissue from adult stem cell sources, are key causes of decreased mobility and strength in aged individuals. If more youthful muscle quality could be restored in old patients they would experience greatly improved quality of life and perhaps even longer lifespans. Satellite cell populations are known to decline sharply by 6-7th decade of life but traditional treatments for sarcopenia, namely exercise intervention, have been shown to exacerbate the degeneration in aged such patients. -

Chelation Therapy
Corporate Medical Policy Chelation Therapy File Name: chelation_therapy Origination: 12/1995 Last CAP Review: 2/2021 Next CAP Review: 2/2022 Last Review: 2/2021 Description of Procedure or Service Chelation therapy is an established treatment for the removal of metal toxins by converting them to a chemically inert form that can be excreted in the urine. Chelation therapy comprises intravenous or oral administration of chelating agents that remove metal ions such as lead, aluminum, mercury, arsenic, zinc, iron, copper, and calcium from the body. Specific chelating agents are used for particular heavy metal toxicities. For example, desferroxamine (not Food and Drug Administration [FDA] approved) is used for patients with iron toxicity, and calcium-ethylenediaminetetraacetic acid (EDTA) is used for patients with lead poisoning. Note that disodium-EDTA is not recommended for acute lead poisoning due to the increased risk of death from hypocalcemia. Another class of chelating agents, called metal protein attenuating compounds (MPACs), is under investigation for the treatment of Alzheimer’s disease, which is associated with the disequilibrium of cerebral metals. Unlike traditional systemic chelators that bind and remove metals from tissues systemically, MPACs have subtle effects on metal homeostasis and abnormal metal interactions. In animal models of Alzheimer’s disease, they promote the solubilization and clearance of β-amyloid protein by binding to its metal-ion complex and also inhibit redox reactions that generate neurotoxic free radicals. MPACs therefore interrupt two putative pathogenic processes of Alzheimer’s disease. However, no MPACs have received FDA approval for treating Alzheimer’s disease. Chelation therapy has also been investigated as a treatment for other indications including atherosclerosis and autism spectrum disorder. -

Chelation of Actinides
UC Berkeley UC Berkeley Previously Published Works Title Chelation of Actinides Permalink https://escholarship.org/uc/item/4b57t174 Author Abergel, RJ Publication Date 2017 DOI 10.1039/9781782623892-00183 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Chapter 6 Chelation of Actinides rebecca J. abergela aChemical Sciences Division, lawrence berkeley National laboratory, One Cyclotron road, berkeley, Ca 94720, USa *e-mail: [email protected] 6.1 The Medical and Public Health Relevance of Actinide Chelation the use of actinides in the civilian industry and defense sectors over the past 60 years has resulted in persistent environmental and health issues, since a large inventory of radionuclides, including actinides such as thorium (th), uranium (U), neptunium (Np), plutonium (pu), americium (am) and curium 1 Downloaded by Lawrence Berkeley National Laboratory on 22/06/2018 20:28:11. (Cm), are generated and released during these activities. Controlled process- Published on 18 October 2016 http://pubs.rsc.org | doi:10.1039/9781782623892-00183 ing and disposal of wastes from the nuclear fuel cycle are the main source of actinide dissemination. however, significant quantities of these radionu- clides have also been dispersed as a consequence of nuclear weapons testing, nuclear power plant accidents, and compromised storage of nuclear materi- als.1 In addition, events of the last fifteen years have heightened public con- cern that actinides may be released as the result of the potential terrorist use of radiological dispersal devices or after a natural disaster affecting nuclear power plants or nuclear material storage sites.2,3 all isotopes of the 15 ele- ments of the actinide series (atomic numbers 89 through 103, Figure 6.1) are radioactive and have the potential to be harmful; the heaviest members, however, are too unstable to be isolated in quantities larger than a few atoms at a time,4 and those elements cited above (U, Np, pu, am, Cm) are the most RSC Metallobiology Series No. -

Effect of Alpha Lipoic Acid on the Blood Cell Count and Iron Kinetics In
Nutr Hosp. 2015;31(2):883-889 ISSN 0212-1611 • CODEN NUHOEQ S.V.R. 318 Original / Farmacia Effect of alpha lipoic acid on the blood cell count and iron kinetics in hypertensive patients Paula Renata Florêncio Mendes, Danielle dos Santos Félix, Paulo César Dantas da Silva, Guêdijany Henrique Pereira and Mônica Oliveira da Silva Simões Dean of Graduate Studies and Research, State University of Paraiba, Campina Grande, Brazil. Abstract EFECTO DEL ÁCIDO LIPOICO EN EL RECUENTO DE SANGRE Y EL METABOLISMO Introduction: The α-lipoic acid (ALA) has been used DE HIERRO EN PACIENTES HIPERTENSOS as a treatment to reduce oxidative damage in Systemic Arterial Hypertension (SAH), but there are no in vivo studies reporting the effect of its mechanism of action on Resumen iron metabolism. Introducción: El Ácido α-Lipóico (ALA) ha sido uti- α Objective: To evaluate the antioxidant effect of -lipoic lizado como recurso terapéutico para reducir daño oxi- acid on Blood cell count (CBC) and iron metabolism in dativo en la Hipertensión Arterial Sistémica (HAS), pero hypertensive subjects with or without anemia. aún no existen estudios in vivo que reporten sobre su me- Method: Double-blind, randomized, placebo-contro- canismo de acción en el metabolismo del hierro. lled clinical trial. The sample consisted of 60 hyperten- Objetivo: Evaluar el efecto antioxidante del ácido Al- sive patients that were randomly divided into treatment fa-Lipóico sobre el hemograma y metabolismo del hierro group (n = 32), receiving 600 mg / day of ALA for twelve en individuos hipertensos con o sin anemia. weeks and control group (n = 28), receiving placebo for Métodos: Estudio clínico doble-ciego, randomizado y the same period. -

Recognizing and Managing Lead and Mercury Poisonings
URGENT CARE Special Section Recognizing and Managing Lead and Mercury Poisonings After iron, which was covered in EM’s May issue, lead and mercury are the two metals most likely to be implicated in heavy metal toxicity syndromes. The authors review the diagnostic considerations and update guidelines for detoxification. hat constitutes a heavy metal? It de- W pends on who you ask. In industrial and environ- mental studies, heavy metals are generally defined as those elements that have high atomic weights—elements with a spe- cific gravity of 5 or higher. A strict chemistry definition classifies ev- Urgent Care Section Urgent Care erything between copper and bis- muth on the periodic table as a heavy metal. Medical usage of the term, however, is much more lib- Inc Researchers, Associates/Photo © 2009 Biphoto eral, encompassing lighter metals Chronic Effects of Lead Poisoning. Dark or bluish discoloration and metalloids that are excluded of the gum-tooth line, known as a “lead line,” may be noted on exam and is the result of a chemical reaction between lead and dental plaque. by other definitions. The list of medically recognized toxic met- als and metalloids includes (but is not limited to) aluminum, arse- poisonings can often result in sig- nic, barium, bismuth, cadmium, nificant morbidity and mortality if cobalt, copper, chromium, gold, unrecognized and inappropriately lead, manganese, mercury, sele- treated. The physician also needs Christian Balmadrid, MD nium, silver, thallium, and zinc.1 to be prepared for the postdiag- Staff Physician Heavy metal or metalloid tox- nostic responsibility of identifying Durham Emergency Physicians Durham Regional Hospital icity is relatively uncommon in possible exposures to the same Durham, North Carolina urgent care centers, but for that toxin within the patient’s family, very reason it is important to workplace, or community that Michael J. -
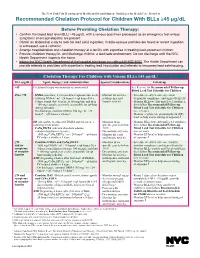
Recommended Chelation Protocol for Children with Blls ≥45 Μg/Dl
The New York City Department of Health and Mental Hygiene Guidelines for Health Care Providers Recommended Chelation Protocol for Children With BLLs ≥45 μg/dL Before Providing Chelation Therapy: • Confirm the blood lead level (BLL) ≥45 μg/dL with a venous specimen processed as an emergency test unless symptoms of encephalopathy are present. • Obtain an abdominal x-ray to look for lead solid ingestion; if radio-opaque particles are found or recent ingestion is witnessed, use a cathartic. • Arrange hospitalization and chelation therapy at a facility with expertise in treating lead-poisoned children. • Provide chelation therapy in, and discharge child to, a lead-safe environment. Do not discharge until the NYC Health Department inspects the home. • Inform the NYC Health Department of the hospital admission by calling 646-632-6002. The Health Department can provide referrals to providers with expertise in treating lead intoxication and referrals to temporary lead-safe housing. Chelation Therapy For Children with Venous BLLs ≥45 μg/dL1 BLL (μg/dL) Agent, Dosage,* and Administration Special Considerations Follow-up <45 Chelation therapy not routinely recommended See Reverse for Recommended Follow-up Blood Lead Test Schedule for Children 45 to <70 • DMSA (succimer, 2,3-meso-dimercaptosuccinic acid) • Monitor for anemia, • Schedule weekly health care visits • 1050 mg DMSA / m2 / 24 hours* ÷ q8 hours PO x neutropenia, and to monitor compliance and signs of toxicity. 5 days; round dose to nearest 100 mg/day, and then hepatic toxicity. • Monitor BLLs weekly until level stabilizes, ÷ 100-mg capsules as evenly as possible for q8-hour then follow Recommended Follow-up dosing schedule. -

Managing Pet Bird Toxicoses J.A
birdtoxic.qxd 6/15/01 4:56 PM Page 23 CLINICIAN’S NOTEBOOK Managing Pet Bird Toxicoses J.A. RICHARDSON, L.A. MURPHY S.A. KHAN AND C. MEANS J.A. Richardson, DVM, Dipl ACFE L.A. Murphy, VMD S.A. Khan, DVM, PhD C. Means, DVM ASPCA Animal Poison Control Center 1717 South Philo Road Suite 36 Urbana, Illinois 61802 [email protected] Jill Richardson received her DVM degree from Tuskegee University in 1994. In 1996, following experience in small animal practices in Tennessee and West Virginia, Dr. Richardson joined the ASPCA Animal Poison Control BIRDS ARE CURIOUS BY NATURE, AND SOME HAZARDOUS OBJECTS Center as a Veterinary Poison may be attractive to them. Birds with free household access are more likely to Information Specialist. be exposed to toxicants. Sources of Toxicoses Affecting Pet Birds Reported to the APCC* 11% 27% 12% Pesticides Cleaning products Acknowledgements Plants Medicines The authors would like to thank 25% 25% Harold and Joyce Hamilton, Other, including heavy metals Cindy Dorner, Morgan Wilson, and Information collected between January 1996 - December 2000. Dr. Tracei Holder and the entire staff of the Animal Emergency Clinic of Champaign County. *The ASPCA Animal Poison Control Center, an operating division of the American Society for Photos: Tom Schaefges Photography the Prevention of Cruelty to Animals (ASPCA), is the only animal-oriented poison control Sidney, Illinois center in North America. It is a unique, emergency hotline providing 24-hour-a-day, [email protected] 7-day-a-week telephone assistance. The Center’s hotline veterinarians can quickly answer questions about toxic chemicals, dangerous plants, products or substances found in everyday surroundings that can prove poisonous or fatal to animals. -

Medical Toxicologists Determine Chelation Therapy Rarely Necessary
Medical Toxicologists Determine Chelation Therapy Rarely Necessary Experts from the American College of Medical Toxicology, the Centers for Disease Control, the Agency for Toxic Substances and Disease Registry, and others met at the CDC to review the current use and misuse of chelation therapy in the United States for the treatment of metal poisoning. It was concluded that incorrect diagnosis of metal poisoning is common, and inappropriate use of chelation therapy is widespread. Phoenix, Arizona (PRWEB) March 30, 2012 -- Medical toxicologists and scientific experts speaking at a recent conference at the Centers for Disease Control in Atlanta criticized the widespread misdiagnosis of poisoning from lead, arsenic, mercury and other metals, and called for strong efforts to decrease the inappropriate use of metal chelation therapy. Chelating agents are medications that may be given to patients to increase elimination of metals from the body. Traditionally chelating agents have been used to treat acute poisoning following a large exposure to a metal such as arsenic, mercury, or lead. In recent years, the administration of chelating agents by some health providers has increased. Although chelating agents such as calcium EDTA, DMSA, and DMPS are medications intended to be prescribed by licensed physicians, they are often sold to patients by health care practitioners or obtained without a prescription over the internet, possibly in violation of federal regulations. It has been estimated that nearly 200,000 people may be treated with chelating agents each year in the United States. These patients are frequently diagnosed with chronic metal poisoning based on a poorly documented environmental exposure, vague clinical findings, and inappropriate diagnostic testing. -

Role of the Intensive Care Unit in the Management of the Poisoned Patient Per Kulling and Hans Persson Swedish Poison Information Centre, Stockholm
Concepts in Toxicology Review Medical Toxicology I: 375·386 (1986) 0112·5966/0090·0375/$06.00/0 © ADIS Press Limited All rights reserved. Role of the Intensive Care Unit in the Management of the Poisoned Patient Per Kulling and Hans Persson Swedish Poison Information Centre, Stockholm Summary By applying a sensible toxicological approach to the general principles ofintensive care, an optimum setting for the treatment ofpoisoning is created. The intensive care unit (ICU) can perform the necessary close observation and monitoring, and thus facilitate rapid detection ofsymptoms, and the institution of early appropriate treatment. Diagnosis may be complex in poisoning and require continuous qualified interpretation of clinical and analytical data. Antidote therapy and treatment to enhance elimination ofthe poison must often be dealt with under careful supervision. The capacity ofthe ICU to counteract various toxic effects in a nonspecific way and to provide optimum symptomatic and supportive care is crucial. However, the ongoing toxic effects on the body must always be considered and allowed to guide symptomatic treatment. Thus, clinical toxicology appears to be a specialised branch of intensive care medicine. Many patients exposed to a poison may, after is of utmost importance for a proper interpretation initial measures like clinical assessment, gastric of clinical and analytical data and an immediate lavage and administration of activated charcoal, be start for necessary therapy. In some cases the ICU managed in general wards. However, to be able to may have a laboratory of its own, offering rapid adequately treat a severely poisoned patient the fa and frequent analysis. Another important aspect of cilities of an intensive care unit (ICU) are often the ICU is that there is generally a low threshold required and the capacity of such units to provide for accepting and utilising new and advanced ther optimum conditions for diagnosis and treatment apeutic methods.