Polish Journal of Natural Sciences Morphometric
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
2 Imxpd 2Mxpd IIS
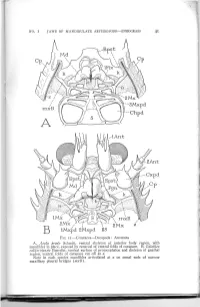
NO. I JAWS OF MANDIBULATE ARTHROPODS SNODGRASS 41 2Mx 2 IMxpd 2Mxpd IIS FIG. II.—Crustacea—Decapoda: Anomura A, Aegla prado Schmitt, ventral skeleton of anterior body region, with mandibles in place, exposed by removal of ventral folds of carapace. B, Galathea calijorniensis Benedict, ventral surface of protocephalon and skeleton of gnathal region, ventral folds of carapace cut off at s. Note in each species mandibles articulated at a on mesal ends of narrow maxillary pleural bridges {mxB). 42 SMITHSONIAN MISCELLANEOUS COLLECTIONS VOL. Il6 FIG. 12.—Crustacea—Decapoda: Anomura. A, Petrolisthes criomerus Stimpson, epistomal region and mandibles, dorsal (interior) view, showing mandibles articulated at a on remnants of maxillary bridges (mxb). B, same, right mandible, ventral. C, Galathea calif orniensis Benedict, muscles of mandibular apodeme arising on carapace. D, same, right mandible, dorsal. E, Pagunts pollicaris Say, left mandible in position of adduc tion, ventral. F, same, same mandible in position of abduction. G, same, distal part of left mandible, showing articulation on epistome. H, Galathea calif orni ensis Benedict, right mandible and its skeletal supports, ventral. ••MMM NO. I JAWS OF MANDIBULATE ARTHROPODS—-SNODGEASS 43 of the mouth folds (fig. i6 E, t), each of which at its distal end divides into a mesal branch that goes into the paragnath (Pgn), and a lateral branch that supports the mandible by means of a small inter vening sclerite (E, H, e). The connection on the mandible is with a process behind the base of the gnathal lobe (figs. 12 D; 15 C, D, F, G; 16 A, B, G, H, d), a feature characteristic of anomuran and brachyuran mandibles; in most cases the metastomal arm makes a direct contact with the mandibular process. -

A New Cavernicolous Species of the Pseudoscorpion Genus Roncus L
Int. J. Speleol. 5 (1973), pp. 127-134. A New Cavernicolous Species of the Pseudoscorpion Genus Roncus L. Koch, 1873 (Neobisiidae, Pseudoscorpiones) from the Balkan Peninsula by Bozidar P.M. CURtlC * The range of the pseudoscorpion subgenus Parablothrlls Beier 1928 (from the genus Roncus L. Koch 1873) extends over the northern Mediterranean, covering a vast zone from Catalonia on the west as far as Thrace on the east. The northern limit of distribution of these false scorpions is situated within the Dolomites and the Alps of Carinthia; the most southern locations of the subgenus were registered on the island of Crete. Eight species of Parablothrus are known to inhabit the Balkan Peninsula which represents an impo;:tant distribution centre of the subgenus (Beier 1963, Helversen 1969); of them, six were found in the Dinaric Karst. The caves of Carniola are thus populated by R. (F:) stussineri (Simon) 1881, and R. (P) anophthalmus (Ellingsen) 1910, R. (P.) cavernicola Beier 1928 and R. (P) vulcanius Beier 1939 are known from Herzegovina. The last species was also collected on some Dalmatian islands. Both Adriatic and Ionian islands are inhabited by two other members of Parablo- thrus, namely R. (P.) insularis Beier 1939 (which was found on the isle of Brae) and R. (P) corcyraeus Beier 1963, the latter living on Corfu. Except for the Dinaric elements of Parablothrus, the Balkan representatives of the subgenus have not been sufficiently studied. In spite of this, one may assume that the differentiation of cave living species of Roncus took place both east and north of the peninsula. -

Morphometric Characteristics of Crayfish Cherax Quadricarinatus from Atokan River, West Sumatera, Indonesia
Eco. Env. & Cons. 26 (4) : 2020; pp. (1787-1792) Copyright@ EM International ISSN 0971–765X Morphometric characteristics of Crayfish Cherax quadricarinatus from Atokan River, West Sumatera, Indonesia Abizar1, Lora Purnamasari1, Widyawati1, Moch Affandi2 and Trisnadi Widyaleksono Catur Putranto2* 1 Study Program of Biology Education, STKIP PGRI Sumatera Barat (College of Teacher Training and Education), Padang, West Sumatra, Indonesia 2Department of Biology, Faculty of Sciences and Technology, Universitas Airlangga, Surabaya, Indonesia (Received 5 April, 2020; Accepted 20 June, 2020) ABSTRACT The objectives of this study were to determine the length–weight relationships (LWRs), sexual dimorphism and condition factors (K) of Cherax quadricarinatus from Atokan River, West Sumatera Indonesia. The sex ratio of C. quadricarinatus was: 1.3:1 (Males:females). Males and females’ crayfish exhibited negative allometric growth (b<3). The length–weight regression was not significantly different between males and females. The condition factor (K) for males and females were 2.85 and 2.32 respectively. There was no significant difference between weight of males and females, however the total length of females was longer than male. There was no significant difference between length and weight of males and females. Carapace width of males and females were not significantly different, meanwhile carapace length of female was longer than the male. The chelae length and chelae width of males and females were not significantly different. This study provides baseline information on morphometric characteristic of C. quadricarinatus in Atokan River, which will be useful for further reference such as management and conservation of the crayfish. Key words : Redclaw, Growth, Sexual dimorphism, Atokan river, West Sumatera. -

Map 53 Bosphorus Compiled by C
Map 53 Bosphorus Compiled by C. Foss, 1995 Introduction (See Map 52) Directory All place names are in Turkey Abbreviation DionByz R. Güngerich (ed.), Dionysii Byzantii Anaplus Bospori, 1927 (reprint, Berlin, 1958) Names Grid Name Period Modern Name / Location Reference Aianteion See Lettered Place Names B2 Aietou Rhynkos Pr. R Yalıköy RE Bosporos 1, col. 753 B2 Akoimeton Mon. L at Eirenaion Janin 1964, 486-87 C3 Akritas Pr. RL Tuzla burnu FOA VIII, 2 A3 Ammoi L E Bakırköy Janin 1964, 443 B2 Amykos HR Beykoz RE Bosporos 1, col. 753 B2 Anaplous?/ L/ Arnavutköy Janin 1964, 468, 477-78 Promotou? L B2 Ancyreum Pr. R Yum burnu RE Bosporos 1, col. 752 B3 [Antigoneia] Ins. Burgaz ada RE Panormos 7 B2 Aphrodysium R Çalı Burnu RE Bosporos 1, col. 751 B2 Archeion R Ortaköy RE Bosporos 1, col. 747 B2 Argyronion RL Macar tabya RE Bosporos 1, col. 752-53 Argyropolis/ See Lettered Place Names Bytharion? Auleon? Sinus See Lettered Water Names Auletes See Lettered Place Names B2 ‘Bacca’ Collis R N Kuruçesme RE Bosporos 1, col. 747 B2 Bacchiae/ C/ Koybaşı RE Bosporos 1, col. 748 Thermemeria HR A2 Barbyses fl. RL Kâgithane deresi RE Bathykolpos See Lettered Water Names B2 *Bathys fl. R Büyükdere DionByz 71; GGM II, 54 B2 Bithynia See Map 52 A2 Blachernai RL Ayvansaray RE; Janin 1964, 57-58 Bolos See Lettered Place Names B2 Boradion L above Kanlıca Janin 1964, 484 B2 Bosphorus RL Bogaziçi RE Bosporos 1; NPauly Bosporos 1 §Bosporos CHRL Bosporion = Phosphorion A2 Bosporios Pr. R Saray burnu RE Βοσπόριος ἄκρα A2 Boukolos Collis R DionByz 25; C. -

A Study on Some Morphological Characteristics of Astacus Leptodactylus (Eschscholtz 1823) in Seven Different Inland Waters in Turkey
J. Black Sea/Mediterranean Environment Vol. 19, No. 2: 190˗205 (2013) RESEARCH ARTICLE A study on some morphological characteristics of Astacus leptodactylus (Eschscholtz 1823) in seven different inland waters in Turkey Tomris Deniz (Bök)1*, Hamdi Aydın2, Celal Ateş3 1Faculty of Fisheries, Istanbul University, Ordu St., No. 200, 34470, Laleli, Istanbul, TURKEY 2Kocaeli University, Gazanfer Bilge Vocational School, 41500 Karamürsel, Kocaeli, TURKEY 3Faculty of Fisheries, Muğla Sıtkı Koçman University, 48000 Kötekli, Muğla, TURKEY *Corresponding author: [email protected] Abstract This study aimed to determine some morphological characteristics of freshwater crayfish (Astacus leptodactylus Eschscholtz 1823) populations in various water resources in Turkey. We present the relationships between total length (TL), carapace length (CL), chelae length (ChL), abdomen length (AL) and total weight (W) for Astacus leptodactylus from three lakes, three dam lakes and an irrigation lake. The values of the exponent b of the length˗weight relationships ranged from 1.0760 to 3.6939 and intercepts from 1.0760 to 3.6939 for combined data. The r2 values ranged from 0.6599 to 0.9561 and relationships were estimated highly significant (P<0.05). Differences in slopes of regression lines between sexes as well as among locations were not significant, tested by ANCOVA. Key words: Crayfish, morphometric characteristics, length˗weight relationship, allometric growth. Introduction Length˗weight relationships have several applications, namely, in fish biology, physiology, ecology and fisheries assessment. According to Andrade and Campos (2002), this is widely used in the analysis of fishery data and particularly useful when sampling large species, mostly because of the difficulty and time required to record weight in the field. -

La Prensa: Único Diario Español E Hispano Americano En Nueva York
Oficinas: Tiempo probable: 245 Canal St., New York. Niil>!ailo Teléfono: Canal 1200. Lluvias fos UNICO DIARÍO ESPAÑOL E HISPANO AMERICANO EN NUEVA YORK. I IV. NUEVA YORK, SABADO 6 DE DICIEMBRE DE 1930. S CENTAVOS. XXV.—NUMERO 4025. , i. MOVIMIENTO ELECCIONA- EL GENERAL URIBURU RIO EN EL SALVADOR PRECISA ECONOMIAS BERENGUER RODEADO DE TODA "'•lio STIMSON ANUNCIA QUE SE IMPEDIRA LA SAN SALVADOR, diciembre Iji'irrl t' » SERAN CASTIGADOS LOS BUENOS AIRES, diciembre 5,(/P). —En el parque Dueñas 'I i'iTjiU,/ 5. — Ei pre»íJenle provi- INMIGRACION DE 135,000 PERSONAS se reunieron hoy en la noche sional, general Uriburu, confe- los partidarios de los candidatos CLASE DE PRECAUCIONES TRAS R renciando noche con alio» presidenciales, generales Clara- con ESTUDIANTES CUBANOS PO funcionarios del gobierno, hizo WASHINGTON, 5 diciembre.—(/P). Apro.ximuda- mount, Lucero y Martinex, del hincapié en la necesidad de im- ingeniero Araujo y del doctor 'ti.'!»,. mcnte 135,000 inmiRrantes ([ue, en condiciones nor- plantar la más estricta econo. Molina, en una tentativa de uni- EL ATENTADO CONTRA SU VIDA mía en la confección del nuevo males hubieran entrado a los Estados Unido.s hasta ficarse. ii' 'lio. pretupuesto. r r><ií|(^ LA ULTIMA l el .'30 de junio de 1931, se verán impedidos de hacer- Miles de persona» asistieron El general Uriburu declaró a la manifestación que siguió, que "es absolutamente impera- lo como resultado de una estricta aplicación de ias en la cual se pronunciaron ca- Se exigirá en lo sucesivo a los periodistas un carnet de tivo ev'tar un déficit, aun a ^oco se les expulsará de la Universidad—Esta per- leyes inmigratorias, s?gún declaró hoy, oficialmente, lidos discursos en favor de los costa de grandes sacrificios" y varios candidatos a la presiden- identificación.—¿Un examen mental?.—Declárase manece cerrada.—Se efectuaron los anunciados leyó un memorándum sobre la el secretario de Estado, Henry L. -

A Study on the Morphometric Characteristics of Astacus Leptodactylus Inhabiting the Thrace Region of Turkey T
Knowledge and Management of Aquatic Ecosystems (2010) 397, 05 http://www.kmae-journal.org c ONEMA, 2010 DOI: 10.1051/kmae/2010021 A study on the morphometric characteristics of Astacus leptodactylus inhabiting the Thrace region of Turkey T. Deniz (Bök) (1),M.M.Harlıoglu˘ (2),M.C.Deval(3) Received March 10, 2010 / Reçu le 10 mars 2010 Revised June 28, 2010 / Révisé le 28 juin 2010 Accepted July 6, 2010 / Accepté le 6 juillet 2010 ABSTRACT Key-words: This study was carried out to determine some morphological characteris- crayfish, tics, and the length-length and length-weight relationships of freshwater morphometric crayfish (A. leptodactylus Eschscholtz 1823) in the area of Tekirdag,˘ in characters, the Thrace region of Turkey. The total length and wet weight for all in- length-weight dividuals ranged from 33 to 156 mm and 0.64 to 96.42 g, respectively. relationship, Although the average length of both sexes was nearly the same, the av- allometric erage weight of male crayfish was higher than that of females. The total growth, length-wet weight relationships for males, females and combined sexes of Thrace region wet weight were found to be: WW = 7.106TL3.293, WW = 2.105TL3.022 and WW = 9.106TL3.224, respectively. Isometric growth for female crayfish and positive allometric growth for male crayfish were observed in all popula- tions. The carapace length, carapace width, and chela length and width increased allometrically with total length (TL) in both sexes. In conclusion, the morphometric relations in A. leptodactylus observed in the present study could provide information for future studies and management plans. -

A Guide to Constantinople
'JUfV ?n fpop A GUIDE CONSTANTINOPLE " The Galata Bridge From Constantinople" By Ooble and Mfflingen [A. & C BlacKn C8546 ^A GUIDE TO CONSTANTINOPLE BY DEMETKIUS COUFOPOULOS FOURTH EDITION ^ LONDON ADAM AND CHARLESCHAI BLACK * 1910 First Edition published September 1S95. Second Edition, October 1S99 Third March 190'2 Edition, ; reprinted January 1900. Fourth Edition. May 1910. PREFACE TO SECOND EDITION The rapid sale of this Guide, and the praise it has received from tourists that have used it, as well as the changes that have occurred since its publication in the city of Constantinople, encourage me to issue a second edition. In preparing this I have care- rally revised the book throughout, re -writing or adding to some passages where necessary. The maps have been brought up to date, and an alpha- betical index has been added D. G. COUFOPOULOS. September 1899. PREFACE TO FIRST EDITION In issuing this Guide to Constantinople let me say at once that it is designed rather for the use of the ordinary sight-seer than of the specialised student. My aim has been to avoid confusing the reader with too great fulness of historical, topo- graphical, or technical details, but rather to fix his attention on salient points, and to convey to him as succinctly as possible such information as is most likely to be of use to one who, without much previous study, wishes to devote a limited time as pleasantly and profitably as may be to the explora- tion of the City and its Environs. In carrying out this aim I hope that my many years' experience as Dragoman in Constantinople will be found to have been not without their use in enabling me to divine the wants of such a traveller as I have indicated. -

Euscorpius. 2013
Three More Species of Euscorpius Confirmed for Greece (Scorpiones: Euscorpiidae) Victor Fet, Michael E. Soleglad, Aristeidis Parmakelis, Panayiota Kotsakiozi & Iasmi Stathi September 2013 — No. 165 Occasional Publications in Scorpiology EDITOR: Victor Fet, Marshall University, ‘[email protected]’ ASSOCIATE EDITOR: Michael E. Soleglad, ‘[email protected]’ Euscorpius is the first research publication completely devoted to scorpions (Arachnida: Scorpiones). Euscorpius takes advantage of the rapidly evolving medium of quick online publication, at the same time maintaining high research standards for the burgeoning field of scorpion science (scorpiology). Euscorpius is an expedient and viable medium for the publication of serious papers in scorpiology, including (but not limited to): systematics, evolution, ecology, biogeography, and general biology of scorpions. Review papers, descriptions of new taxa, faunistic surveys, lists of museum collections, and book reviews are welcome. Derivatio Nominis The name Euscorpius Thorell, 1876 refers to the most common genus of scorpions in the Mediterranean region and southern Europe (family Euscorpiidae). Euscorpius is located at: http://www.science.marshall.edu/fet/Euscorpius (Marshall University, Huntington, West Virginia 25755-2510, USA) ICZN COMPLIANCE OF ELECTRONIC PUBLICATIONS: Electronic (“e-only”) publications are fully compliant with ICZN (International Code of Zoological Nomenclature) (i.e. for the purposes of new names and new nomenclatural acts) when properly archived and registered. All Euscorpius issues starting from No. 156 (2013) are archived in two electronic archives: Biotaxa, http://biotaxa.org/Euscorpius (ICZN-approved and ZooBank-enabled) Marshall Digital Scholar, http://mds.marshall.edu/euscorpius/. (This website also archives all Euscorpius issues previously published on CD-ROMs.) Between 2000 and 2013, ICZN did not accept online texts as "published work" (Article 9.8). -

The Burial Custom of Cremation and the Warrior Order Of
A Dignified Passage through the Gates of Hades The Burial Custom of Cremation and the Warrior Order of Ancient Eleutherna Anagnostis P. Agelarakis Access Archaeology o hae pre rc s A s A y c g c e o l s o s e A a r c Ah Archaeopress Publishing Ltd Gordon House 276 Banbury Road Oxford OX2 7ED www.archaeopress.com ISBN 978 1 78491 383 0 ISBN 978 1 78491 384 7 (e-Pdf) © Archaeopress and A P Agelarakis 2016 All rights reserved. No part of this book may be reproduced or transmitted, in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior written permission of the copyright owners. Contents A Dignified Passage through the Gates of Hades: The Burial Custom of Cremation and the Warrior Order of Ancient Eleutherna �����������������������������������������������������������������������������������������������������������1 Prologue ..................................................................................................................................................... 1 Introduction ................................................................................................................................................ 1 Anthropological insights on monumental tomb A1K1 ............................................................................... 2 The warrior order of ancient Eleutherna .................................................................................................... 4 Deciphering conferred funerary whispers ................................................................................................. -
Spring 2004 No
ILLINOIS NATURAL HISTORY R e p o r t s SURVE Y Spring 2004 No. 379 I N S I D E Prospects for Biological Control of Teasel in Highlights of the Long Illinois Term Resource Monitoring Program at the INHS Some plants that arrive in Illinois included cutting and Great Rivers Field from exotic homes lack natural herbiciding, but Station enemies to keep their popula- costs of labor and 2 tions in check and become inva- chemicals, as well sive. The teasel species as harm to nontarget INHS Researcher (Dipsacus fullonum [common plants from Named a Fellow by The teasel] and D. laciniatus [cut- overspray, have re- Wildlife Society leafed teasel]) are examples of sulted in reduced 4 such exotic plants. These Old effectiveness of World plants have been in North these methods. Bio- Channelization, a America since the 1800s and in logical control is one Major Factor Influenc- Illinois for a number of years. of the few remaining ing Stream Condition The first records of D. fullonum approaches possible. in Illinois (referred to as D. sylvestris) in Staff from the 5 the Illinois Natural History Sur- INHS Center for Critical Trends vey (INHS) Herbarium date to Ecological Entomol- Assessment Program 1934, but teasel recently has be- ogy have developed Web Page: come more visible along road- a partnership with AWindow into Illinois sides, pastures, and untilled scientists at the Habitats lands. USDA-ARS Euro- 8 Mowing is considered to be pean Biological one of the primary means of Control Laboratory Transmission Cycle of spreading teasel, and changes in near Montpellier, Neospora caninum: A mowing patterns may be respon- France, Millikin Brian Rector of the USDA-ARS European University, and the Biological Control Laboratory is dwarfed by a Single-cell Parasite at sible for the increased visibility. -

On the Origin, Growth, and Arrangement of Sponge-Spicules: a Study in Symbiosis
On the Origin, Growth, and arrangement of Sponge-spicules: A study in Symbiosis. By Arthur Dendy, D.Sc, F.R.S., Late Professor of Zoology in the University of London and Fellow of King's College. With Plates 1, 2, and 3, and 16 Text-figures. CONTENTS. PAGE 1. INTRODUCTORY REMARKS ....... 2 2. SPEGIAL OBSERVATIONS ....... 0 (d) The Stephanotyles of Sceptrospongia coronata . 0 (b) The suppressed Triaenes of Stelletta haeckeli . ft (c) The Formation of Arnphitriaenes by incomplete Twinning 12 {d) The Development of adventitious Rays . .IS (e) The Formation of nodal Whorls in Discorhabds . 18 (/) A revised Account of the Development of the Anisodisco- rhabd in Latrunculia bocagei .... 27 3. GENERAL CONCLUSIONS AS TO THE ORIGIN AND GROWTH OF SILICEOUS SPONGE-SPICULES . .... 36 (a) The primary co-operating Agents . 3(> (6) The Influence of the primary Protorhabd upon the Form of the Spicule 3!> (c) The Influence of the Mode of Division of the original Scleroplastid . .41 (a) The Formation of radiate Spicules . .41 (|S) Variation in the number of primary Rays . 43 (y) The Formation of Dragmata and Rosettes . 44 (d) The Influence of adventitious Growing Points upon the Form of the Spicule 40 (a) Adventitious Growing Points associated with Protorhabds 40 (|3) Adventitious Growing Points without Protorhabd Formation ....... 47 NO. 277 B 2 ARTHUR DENDY PAGE (e) Mechanical Influences affecting the Form of the Spicule . 49 (n) The Conversion of dermal Spicules into Plates . 49 (j9) The Mechanical Influence of the so-called Mother- cell 50 (y) Other Mechanical Influences . 51 4. COMPARISON OF THE DEVELOPMENT OF CALCAREOUS SPONGE- SPICULES 52 5.