Venezuelan Macronyssidae (Acarina: Mesostigmata)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Predatory Mite (Acari, Parasitiformes: Mesostigmata (Gamasina); Acariformes: Prostigmata) Community in Strawberry Agrocenosis
Acta Universitatis Latviensis, Biology, 2004, Vol. 676, pp. 87–95 The predatory mite (Acari, Parasitiformes: Mesostigmata (Gamasina); Acariformes: Prostigmata) community in strawberry agrocenosis Valentîna Petrova*, Ineta Salmane, Zigrîda Çudare Institute of Biology, University of Latvia, Miera 3, Salaspils LV-2169, Latvia *Corresponding author, E-mail: [email protected]. Abstract Altogether 37 predatory mite species from 14 families (Parasitiformes and Acariformes) were collected using leaf sampling and pit-fall trapping in strawberry fi elds (1997 - 2001). Thirty- six were recorded on strawberries for the fi rst time in Latvia. Two species, Paragarmania mali (Oud.) (Aceosejidae) and Eugamasus crassitarsis (Hal.) (Parasitidae) were new for the fauna of Latvia. The most abundant predatory mite families (species) collected from strawberry leaves were Phytoseiidae (Amblyseius cucumeris Oud., A. aurescens A.-H., A. bicaudus Wainst., A. herbarius Wainst.) and Anystidae (Anystis baccarum L.); from pit-fall traps – Parasitidae (Poecilochirus necrophori Vitz. and Parasitus lunaris Berl.), Aceosejidae (Leioseius semiscissus Berl.) and Macrochelidae (Macrocheles glaber Müll). Key words: agrocenosis, diversity, predatory mites, strawberry. Introduction Predatory mites play an important ecological role in terrestrial ecosystems and they are increasingly being used in management for biocontrol of pest mites, thrips and nematodes (Easterbrook 1992; Wright, Chambers 1994; Croft et al. 1998; Cuthbertson et al. 2003). Many of these mites have a major infl uence on nutrient cycling, as they are predators on other arthropods (Santos 1985; Karg 1993; Koehler 1999). In total, investigations of mite fauna in Latvia were made by Grube (1859), who found 28 species, Eglītis (1954) – 50 species, Kuznetsov and Petrov (1984) – 85 species, Lapiņa (1988) – 207 species, and Salmane (2001) – 247 species. -

Gamasid Mites
NATIONAL RESEARCH TOMSK STATE UNIVERSITY BIOLOGICAL INSTITUTE RUSSIAN ACADEMY OF SCIENCE ZOOLOGICAL INSTITUTE M.V. Orlova, M.K. Stanyukovich, O.L. Orlov GAMASID MITES (MESOSTIGMATA: GAMASINA) PARASITIZING BATS (CHIROPTERA: RHINOLOPHIDAE, VESPERTILIONIDAE, MOLOSSIDAE) OF PALAEARCTIC BOREAL ZONE (RUSSIA AND ADJACENT COUNTRIES) Scientific editor Andrey S. Babenko, Doctor of Science, professor, National Research Tomsk State University Tomsk Publishing House of Tomsk State University 2015 UDK 576.89:599.4 BBK E693.36+E083 Orlova M.V., Stanyukovich M.K., Orlov O.L. Gamasid mites (Mesostigmata: Gamasina) associated with bats (Chiroptera: Vespertilionidae, Rhinolophidae, Molossidae) of boreal Palaearctic zone (Russia and adjacent countries) / Scientific editor A.S. Babenko. – Tomsk : Publishing House of Tomsk State University, 2015. – 150 р. ISBN 978-5-94621-523-7 Bat gamasid mites is a highly specialized ectoparasite group which is of great interest due to strong isolation and other unique features of their hosts (the ability to fly, long distance migration, long-term hibernation). The book summarizes the results of almost 60 years of research and is the most complete summary of data on bat gamasid mites taxonomy, biology, ecol- ogy. It contains the first detailed description of bat wintering experience in sev- eral regions of the boreal Palaearctic. The book is addressed to zoologists, ecologists, experts in environmental protection and biodiversity conservation, students and teachers of biology, vet- erinary science and medicine. UDK 576.89:599.4 -

Ectoparasites of Bats in Mongolia, Part 2 (Ischnopsyllidae, Nycteribiidae, Cimicidae and Acari) Ingo Scheffler University of Potsdam, [email protected]
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Erforschung biologischer Ressourcen der Mongolei Institut für Biologie der Martin-Luther-Universität / Exploration into the Biological Resources of Halle-Wittenberg Mongolia, ISSN 0440-1298 2012 Ectoparasites of Bats in Mongolia, Part 2 (Ischnopsyllidae, Nycteribiidae, Cimicidae and Acari) Ingo Scheffler University of Potsdam, [email protected] Dietrich Dolch Radensleben, Germany Jargalsaikhan Ariunbold Mongolian State University of Education Annegret Stubbe Martin-Luther Universität, [email protected] Andreas Abraham University of Potsdam FSeoe nelloxtw pa thige fors aaddndition addal aitutionhorsal works at: http://digitalcommons.unl.edu/biolmongol Part of the Asian Studies Commons, Biodiversity Commons, Environmental Sciences Commons, Nature and Society Relations Commons, Other Animal Sciences Commons, Parasitology Commons, and the Zoology Commons Scheffler, Ingo; Dolch, Dietrich; Ariunbold, Jargalsaikhan; Stubbe, Annegret; Abraham, Andreas; and Thiele, Klaus, "Ectoparasites of Bats in Mongolia, Part 2 (Ischnopsyllidae, Nycteribiidae, Cimicidae and Acari)" (2012). Erforschung biologischer Ressourcen der Mongolei / Exploration into the Biological Resources of Mongolia, ISSN 0440-1298. 16. http://digitalcommons.unl.edu/biolmongol/16 This Article is brought to you for free and open access by the Institut für Biologie der Martin-Luther-Universität Halle-Wittenberg at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Erforschung biologischer Ressourcen der Mongolei / Exploration into the Biological Resources of Mongolia, ISSN 0440-1298 by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Ingo Scheffler, Dietrich Dolch, Jargalsaikhan Ariunbold, Annegret Stubbe, Andreas Abraham, and Klaus Thiele This article is available at DigitalCommons@University of Nebraska - Lincoln: http://digitalcommons.unl.edu/biolmongol/16 Copyright 2012, Martin-Luther-Universität Halle Wittenberg, Halle (Saale). -

© 2004 by Steven James Presley
© 2004 by Steven James Presley ACKNOWLEDGMENTS Foremost, I thank my major professor, Dr. Michael Willig, for his continual support, encouragement, criticism, and enthusiasm. Mike provided many and varied opportunities for me to grow as a researcher, thinker, educator, and person; hopefully those opportunities were not wasted. Under his guidance I have become a well-rounded scientist, critical thinker, proficient writer, and capable statistician. I am indebted to my committee, Drs. Don Gettinger, Mark McGinley, Daryl Moorhead, Robert Owen, and Richard Strauss. Each has contributed significantly to my growth as a scientist and this dissertation would be lacking if not for their collective guidance. I also thank many faculty members of Texas Tech University, who have provided guidance and enriched my doctoral experience, including Drs. Ray Jackson, Kent Rylander, Michael San Francisco, Charlie Werth, Gene Wilde, and John Zak. I thank Dr. Michael Dini for helping to develop my skills as an instructor. Many fellow graduate students made my time at Texas Tech enjoyable and productive. Stephen Cox was influential in my early development as a doctoral student; we had many discussions over a well-packed bowl that broadened my outlook of the world and biology. Christopher Bloch has been an invaluable office mate during the course of the analysis and writing of my dissertation, being a patient listener and sounding board for my ideas. In addition, I am indebted to Richard Stevens, Celia López-González, Carl Dick, Joel Brant, Chris Higgins, P. Marcos Gorreson, Ed Sobek, Michael Cramer, Kate Lyons, Michelle Secrest, Diane Hall, Brian Croyle, Javier Alvarez, Jeff Roberts, Don Yee, Carla Guthrie, and Kelly Johnson for their friendship, guidance, and support during various epochs of my doctoral studies. -
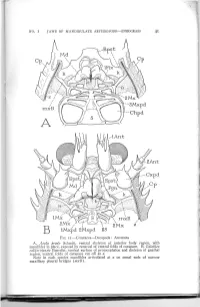
2 Imxpd 2Mxpd IIS
NO. I JAWS OF MANDIBULATE ARTHROPODS SNODGRASS 41 2Mx 2 IMxpd 2Mxpd IIS FIG. II.—Crustacea—Decapoda: Anomura A, Aegla prado Schmitt, ventral skeleton of anterior body region, with mandibles in place, exposed by removal of ventral folds of carapace. B, Galathea calijorniensis Benedict, ventral surface of protocephalon and skeleton of gnathal region, ventral folds of carapace cut off at s. Note in each species mandibles articulated at a on mesal ends of narrow maxillary pleural bridges {mxB). 42 SMITHSONIAN MISCELLANEOUS COLLECTIONS VOL. Il6 FIG. 12.—Crustacea—Decapoda: Anomura. A, Petrolisthes criomerus Stimpson, epistomal region and mandibles, dorsal (interior) view, showing mandibles articulated at a on remnants of maxillary bridges (mxb). B, same, right mandible, ventral. C, Galathea calif orniensis Benedict, muscles of mandibular apodeme arising on carapace. D, same, right mandible, dorsal. E, Pagunts pollicaris Say, left mandible in position of adduc tion, ventral. F, same, same mandible in position of abduction. G, same, distal part of left mandible, showing articulation on epistome. H, Galathea calif orni ensis Benedict, right mandible and its skeletal supports, ventral. ••MMM NO. I JAWS OF MANDIBULATE ARTHROPODS—-SNODGEASS 43 of the mouth folds (fig. i6 E, t), each of which at its distal end divides into a mesal branch that goes into the paragnath (Pgn), and a lateral branch that supports the mandible by means of a small inter vening sclerite (E, H, e). The connection on the mandible is with a process behind the base of the gnathal lobe (figs. 12 D; 15 C, D, F, G; 16 A, B, G, H, d), a feature characteristic of anomuran and brachyuran mandibles; in most cases the metastomal arm makes a direct contact with the mandibular process. -

Ornithonyssus Sylviarum (Acari: Macronyssidae)
Ciência Rural,Ornithonyssus Santa sylviarumMaria, v.50:7, (Acari: Macronyssidaee20190358, )2020 parasitism among poultry farm workers http://doi.org/10.1590/0103-8478cr20190358 in Minas Gerais state, Brazil. 1 ISSNe 1678-4596 PARASITOLOGY Ornithonyssus sylviarum (Acari: Macronyssidae) parasitism among poultry farm workers in Minas Gerais state, Brazil Cristina Mara Teixeira1 Tiago Mendonça de Oliveira2* Amanda Soriano-Araújo3 Leandro do Carmo Rezende4 Paulo Roberto de Oliveira2† Lucas Maciel Cunha5 Nelson Rodrigo da Silva Martins2 1Ministério da Agricultura Pecuária e Abastecimento (DIPOA), Brasília, DF, Brasil. 2Departamento de Medicina Veterinária Preventiva da Escola de Veterinária da Universidade Federal de Minas Gerais (UFMG), 31270-901, Belo Horizonte, MG, Brasil. E-mail: [email protected]. *Corresponding author. †In memoriam. 3Instituto Federal de Minas Gerais (IFMG), Bambuí, MG, Brasil. 4Laboratório Federal de Defesa Agropecuária (LFDA), Pedro Leopoldo, MG, Brasil. 5Fundação Ezequiel Dias, Belo Horizonte, MG, Brasil. ABSTRACT: Ornithonyssus sylviarum is a hematophagous mite present in wild, domestic, and synanthropic birds. However, this mite can affect several vertebrate hosts, including humans, leading to dermatitis, pruritus, allergic reactions, and papular skin lesions. This study evaluated the epidemiological characteristics of O. sylviarum attacks on poultry workers, including data on laying hens, infrastructure and management of hen houses, and reports of attacks by hematophagous mites. In addition, a case of mite attack on a farm worker on a laying farm in the Midwest region in Minas Gerais is presented. It was found that 60.7% farm workers reported attacks by hematophagous mites. Correspondence analysis showed an association between reports of mite attacks in humans with (1) presence of O. sylviarum in the hen house, (2) manual removal of manure by employees, and (3) history of acaricide use. -

Two New Species of Gaeolaelaps (Acari: Mesostigmata: Laelapidae)
Zootaxa 3861 (6): 501–530 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3861.6.1 http://zoobank.org/urn:lsid:zoobank.org:pub:60747583-DF72-45C4-AE53-662C1CE2429C Two new species of Gaeolaelaps (Acari: Mesostigmata: Laelapidae) from Iran, with a revised generic concept and notes on significant morphological characters in the genus SHAHROOZ KAZEMI1, ASMA RAJAEI2 & FRÉDÉRIC BEAULIEU3 1Department of Biodiversity, Institute of Science and High Technology and Environmental Sciences, Graduate University of Advanced Technology, Kerman, Iran. E-mail: [email protected] 2Department of Plant Protection, College of Agriculture, University of Agricultural Sciences and Natural Resources, Gorgan, Iran. E-mail: [email protected] 3Canadian National Collection of Insects, Arachnids and Nematodes, Agriculture and Agri-Food Canada, 960 Carling avenue, Ottawa, ON K1A 0C6, Canada. E-mail: [email protected] Abstract Two new species of laelapid mites of the genus Gaeolaelaps Evans & Till are described based on adult females collected from soil and litter in Kerman Province, southeastern Iran, and Mazandaran Province, northern Iran. Gaeolaelaps jondis- hapouri Nemati & Kavianpour is redescribed based on the holotype and additional specimens collected in southeastern Iran. The concept of the genus is revised to incorporate some atypical characters of recently described species. Finally, some morphological attributes with -

Records of the Hawaii Biological Survey for 1996
Records of the Hawaii Biological Survey for 1996. Bishop Museum Occasional Papers 49, 71 p. (1997) RECORDS OF THE HAWAII BIOLOGICAL SURVEY FOR 1996 Part 2: Notes1 This is the second of 2 parts to the Records of the Hawaii Biological Survey for 1996 and contains the notes on Hawaiian species of protists, fungi, plants, and animals includ- ing new state and island records, range extensions, and other information. Larger, more comprehensive treatments and papers describing new taxa are treated in the first part of this Records [Bishop Museum Occasional Papers 48]. Foraminifera of Hawaii: Literature Survey THOMAS A. BURCH & BEATRICE L. BURCH (Research Associates in Zoology, Hawaii Biological Survey, Bishop Museum, 1525 Bernice Street, Honolulu, HI 96817, USA) The result of a compilation of a checklist of Foraminifera of the Hawaiian Islands is a list of 755 taxa reported in the literature below. The entire list is planned to be published as a Bishop Museum Technical Report. This list also includes other names that have been applied to Hawaiian foraminiferans. Loeblich & Tappan (1994) and Jones (1994) dis- agree about which names should be used; therefore, each is cross referenced to the other. Literature Cited Bagg, R.M., Jr. 1980. Foraminifera collected near the Hawaiian Islands by the Steamer Albatross in 1902. Proc. U.S. Natl. Mus. 34(1603): 113–73. Barker, R.W. 1960. Taxonomic notes on the species figured by H. B. Brady in his report on the Foraminifera dredged by HMS Challenger during the years 1873–1876. Soc. Econ. Paleontol. Mineral. Spec. Publ. 9, 239 p. Belford, D.J. -

Tropical Fowl Mite, Ornithonyssus Bursa (Berlese) (Arachnida: Acari: Macronyssidae)1 H
EENY-297 Tropical Fowl Mite, Ornithonyssus bursa (Berlese) (Arachnida: Acari: Macronyssidae)1 H. A. Denmark and H. L. Cromroy2 Introduction The tropical fowl mite, commonly found on birds, has become a pest to people in areas of high bird populations or where birds are allowed to roost on roofs, around the eaves of homes, and office buildings. Nesting birds are the worst offenders. After the birds abandon their nests, the mites move into the building through windows, doors, and vents and bite the occupants. The bite is irritating, and some individuals react to the bite with prolonged itching and painful dermatitis. Several to many reports are received each year of mites invading homes. The mites are usually the tropical fowl mite found in the central and southern areas of the state. The northern fowl mite, Ornithonyssus sylviarum (Canestrini and Fan- zago), a close relative, is also found in Florida. Synonyms Leiognathus bursa Berlese (1888) Figure 1. Scanning electron microscope (SEM) photograph showing Liponyssus bursa Hirst (1916) ventral view of the tropical fowl mite, Ornithonyssus bursa (Berlese). Ornithonyssus bursa Sambon (1928) Credits: H. L. Cromroy, UF/IFAS Distribution • Australia—New South Wales, Queensland, South Australia • Africa—Egypt, Nigeria, Malawi, Republic of South Africa • Central America—Canal Zone • Asia—China, India, Thailand. Indonesia - Java, Mauritius • Islands of the Indian Ocean—Comoro Islands, Zanzibar 1. This document is EENY-297, one of a series of the Department of Entomology and Nematology, UF/IFAS Extension. Original publication date July 2003. Revised November 2011 and November 2015. Reviewed October 2018. Visit the EDIS website at http://edis.ifas.ufl.edu. -

A New Cavernicolous Species of the Pseudoscorpion Genus Roncus L
Int. J. Speleol. 5 (1973), pp. 127-134. A New Cavernicolous Species of the Pseudoscorpion Genus Roncus L. Koch, 1873 (Neobisiidae, Pseudoscorpiones) from the Balkan Peninsula by Bozidar P.M. CURtlC * The range of the pseudoscorpion subgenus Parablothrlls Beier 1928 (from the genus Roncus L. Koch 1873) extends over the northern Mediterranean, covering a vast zone from Catalonia on the west as far as Thrace on the east. The northern limit of distribution of these false scorpions is situated within the Dolomites and the Alps of Carinthia; the most southern locations of the subgenus were registered on the island of Crete. Eight species of Parablothrus are known to inhabit the Balkan Peninsula which represents an impo;:tant distribution centre of the subgenus (Beier 1963, Helversen 1969); of them, six were found in the Dinaric Karst. The caves of Carniola are thus populated by R. (F:) stussineri (Simon) 1881, and R. (P) anophthalmus (Ellingsen) 1910, R. (P.) cavernicola Beier 1928 and R. (P) vulcanius Beier 1939 are known from Herzegovina. The last species was also collected on some Dalmatian islands. Both Adriatic and Ionian islands are inhabited by two other members of Parablo- thrus, namely R. (P.) insularis Beier 1939 (which was found on the isle of Brae) and R. (P) corcyraeus Beier 1963, the latter living on Corfu. Except for the Dinaric elements of Parablothrus, the Balkan representatives of the subgenus have not been sufficiently studied. In spite of this, one may assume that the differentiation of cave living species of Roncus took place both east and north of the peninsula. -

THÈSE Docteur L'institut Des Sciences Et Industries Du Vivant Et De L
N° /__/__/__/__/__/__/__/__/__/__/ THÈSE pour obtenir le grade de Docteur de l’Institut des Sciences et Industries du Vivant et de l’Environnement (Agro Paris Tech) Spécialité : Biologie de l’Evolution et Ecologie présentée et soutenue publiquement par ROY Lise le 11 septembre 2009 11 septembre 2009 ECOLOGIE EVOLUTIVE D’UN GENRE D’ACARIEN HEMATOPHAGE : APPROCHE PHYLOGENETIQUE DES DELIMITATIONS INTERSPECIFIQUES ET CARACTERISATION COMPARATIVE DES POPULATIONS DE CINQ ESPECES DU GENRE DERMANYSSUS (ACARI : MESOSTIGMATA) Directeur de thèse : Claude Marie CHAUVE Codirecteur de thèse : Thierry BURONFOSSE Travail réalisé : Ecole Nationale Vétérinaire de Lyon, Laboratoire de Parasitologie et Maladies parasitaires, F-69280 Marcy-L’Etoile Devant le jury : M. Jacques GUILLOT, PR, Ecole Nationale Vétérinaire de Maisons-Alfort (ENVA).…………...Président M. Mark MARAUN, PD, J.F. Blumenbach Institute of Zoology and Anthropology...…………...Rapporteur Mme Maria NAVAJAS, DR, Institut National de la Recherche Agronomique (INRA)..………... Rapporteur M. Roland ALLEMAND, CR, Centre national de la recherche scientifique (CNRS).……………Examinateur M. Thierry BOURGOIN, PR, Muséum National d’Histoire Naturelle (MNHN)......….... ………….Examinateur M. Thierry BURONFOSSE, MC, Ecole Nationale Vétérinaire de Lyon (ENVL)...……………..… Examinateur Mme Claude Marie CHAUVE, PR, Ecole Nationale Vétérinaire de Lyon (ENVL)…...………….. Examinateur L’Institut des Sciences et Industries du Vivant et de l’Environnement (Agro Paris Tech) est un Grand Etablissement dépendant du Ministère de l’Agriculture et de la Pêche, composé de l’INA PG, de l’ENGREF et de l’ENSIA (décret n° 2006-1592 du 13 décembre 2006) Résumé Les acariens microprédateurs du genre Dermanyssus (espèces du groupe gallinae), inféodés aux oiseaux, représentent un modèle pour l'étude d'association lâche particulièrement intéressant : ces arthropodes aptères font partie intégrante du microécosystème du nid (repas de sang aussi rapide que celui du moustique) et leurs hôtes sont ailés. -

Mesostigmata: Dermanyssidae), in Laboratory and Field Trials*
35-Liebisch et al-AF:35-Liebisch et al-AF 11/25/11 2:52 AM Page 282 Zoosymposia 6: 282–287 (2011) ISSN 1178-9905 (print edition) www.mapress.com/zoosymposia/ ZOOSYMPOSIA Copyright © 2011 . Magnolia Press ISSN 1178-9913 (online edition) Efficacy of spinosad against the poultry red mite, Dermanyssus gallinae (Mesostigmata: Dermanyssidae), in laboratory and field trials* GABRIELE LIEBISCH, RICHARD HACK & GOSSE SMID Laboratorium für klinische Diagnostik ZeckLab, Up’n Kampe 3, D-30938, Germany * In: Moraes, G.J. de & Proctor, H. (eds) Acarology XIII: Proceedings of the International Congress. Zoosymposia, 6, 1–304. Abstract The poultry red mite, Dermanyssus gallinae (De Geer), is the most economically important ectoparasite in poultry houses in many countries around the world. The lack of efficacy of commercial products in its control results from poor application and/or from its resistance to active ingredients. A new insecticide, spinosad, was tested against mobi - le stages of the red mite in laboratory and field populations. Laboratory trials showed increasing efficacy five days (adults) and six days (nymphs) after exposure. Laboratory results were confirmed in a field trial conducted under com - mercial conditions. The trial was conducted in three separate houses on farms with high natural mite populations. The first and second houses were sprayed with concentrations of 2,000 and 4,000 ppm of spinosad (2 and 4 g/L), whereas the third house was used as an untreated control. Spraying was conducted using a sprayer (Stadikopumpe VA AR 252- 200 LE, Dinklage, Germany) with a 200 L reservoir with permanent stirring, a 50 m long flexible tube with a double jet system, and jet size of 0.08 mm.