Exploring the Water Uptake and Release of Mars-Relevant Salt and Surface Analogs Through Raman Microscopy by Katherine M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Vertical Files Anderson Reading Room Center for Southwest Research Zimmerman Library
“A” – biographical Abiquiu, NM GUIDE TO THE GENERAL VERTICAL FILES ANDERSON READING ROOM CENTER FOR SOUTHWEST RESEARCH ZIMMERMAN LIBRARY (See UNM Archives Vertical Files http://rmoa.unm.edu/docviewer.php?docId=nmuunmverticalfiles.xml) FOLDER HEADINGS “A” – biographical Alpha folders contain clippings about various misc. individuals, artists, writers, etc, whose names begin with “A.” Alpha folders exist for most letters of the alphabet. Abbey, Edward – author Abeita, Jim – artist – Navajo Abell, Bertha M. – first Anglo born near Albuquerque Abeyta / Abeita – biographical information of people with this surname Abeyta, Tony – painter - Navajo Abiquiu, NM – General – Catholic – Christ in the Desert Monastery – Dam and Reservoir Abo Pass - history. See also Salinas National Monument Abousleman – biographical information of people with this surname Afghanistan War – NM – See also Iraq War Abousleman – biographical information of people with this surname Abrams, Jonathan – art collector Abreu, Margaret Silva – author: Hispanic, folklore, foods Abruzzo, Ben – balloonist. See also Ballooning, Albuquerque Balloon Fiesta Acequias – ditches (canoas, ground wáter, surface wáter, puming, water rights (See also Land Grants; Rio Grande Valley; Water; and Santa Fe - Acequia Madre) Acequias – Albuquerque, map 2005-2006 – ditch system in city Acequias – Colorado (San Luis) Ackerman, Mae N. – Masonic leader Acoma Pueblo - Sky City. See also Indian gaming. See also Pueblos – General; and Onate, Juan de Acuff, Mark – newspaper editor – NM Independent and -

Martian Crater Morphology
ANALYSIS OF THE DEPTH-DIAMETER RELATIONSHIP OF MARTIAN CRATERS A Capstone Experience Thesis Presented by Jared Howenstine Completion Date: May 2006 Approved By: Professor M. Darby Dyar, Astronomy Professor Christopher Condit, Geology Professor Judith Young, Astronomy Abstract Title: Analysis of the Depth-Diameter Relationship of Martian Craters Author: Jared Howenstine, Astronomy Approved By: Judith Young, Astronomy Approved By: M. Darby Dyar, Astronomy Approved By: Christopher Condit, Geology CE Type: Departmental Honors Project Using a gridded version of maritan topography with the computer program Gridview, this project studied the depth-diameter relationship of martian impact craters. The work encompasses 361 profiles of impacts with diameters larger than 15 kilometers and is a continuation of work that was started at the Lunar and Planetary Institute in Houston, Texas under the guidance of Dr. Walter S. Keifer. Using the most ‘pristine,’ or deepest craters in the data a depth-diameter relationship was determined: d = 0.610D 0.327 , where d is the depth of the crater and D is the diameter of the crater, both in kilometers. This relationship can then be used to estimate the theoretical depth of any impact radius, and therefore can be used to estimate the pristine shape of the crater. With a depth-diameter ratio for a particular crater, the measured depth can then be compared to this theoretical value and an estimate of the amount of material within the crater, or fill, can then be calculated. The data includes 140 named impact craters, 3 basins, and 218 other impacts. The named data encompasses all named impact structures of greater than 100 kilometers in diameter. -

March 21–25, 2016
FORTY-SEVENTH LUNAR AND PLANETARY SCIENCE CONFERENCE PROGRAM OF TECHNICAL SESSIONS MARCH 21–25, 2016 The Woodlands Waterway Marriott Hotel and Convention Center The Woodlands, Texas INSTITUTIONAL SUPPORT Universities Space Research Association Lunar and Planetary Institute National Aeronautics and Space Administration CONFERENCE CO-CHAIRS Stephen Mackwell, Lunar and Planetary Institute Eileen Stansbery, NASA Johnson Space Center PROGRAM COMMITTEE CHAIRS David Draper, NASA Johnson Space Center Walter Kiefer, Lunar and Planetary Institute PROGRAM COMMITTEE P. Doug Archer, NASA Johnson Space Center Nicolas LeCorvec, Lunar and Planetary Institute Katherine Bermingham, University of Maryland Yo Matsubara, Smithsonian Institute Janice Bishop, SETI and NASA Ames Research Center Francis McCubbin, NASA Johnson Space Center Jeremy Boyce, University of California, Los Angeles Andrew Needham, Carnegie Institution of Washington Lisa Danielson, NASA Johnson Space Center Lan-Anh Nguyen, NASA Johnson Space Center Deepak Dhingra, University of Idaho Paul Niles, NASA Johnson Space Center Stephen Elardo, Carnegie Institution of Washington Dorothy Oehler, NASA Johnson Space Center Marc Fries, NASA Johnson Space Center D. Alex Patthoff, Jet Propulsion Laboratory Cyrena Goodrich, Lunar and Planetary Institute Elizabeth Rampe, Aerodyne Industries, Jacobs JETS at John Gruener, NASA Johnson Space Center NASA Johnson Space Center Justin Hagerty, U.S. Geological Survey Carol Raymond, Jet Propulsion Laboratory Lindsay Hays, Jet Propulsion Laboratory Paul Schenk, -

Santa Fe National Historic Trail: Special History Study
Santa Fe National Historic Trail: Special History Study SANTE FE Special History Study COMERCIANTES, ARRIEROS, Y PEONES: THE HISPANOS AND THE SANTA FE TRADE (Merchants, Muleteers, and Peons) Special History Study Santa Fe National Historic Trail by Susan Calafate Boyle Southwest Cultural Resources Center Professional Papers No. 54 Division of History Southwest Region National Park Service 1994 TABLE OF CONTENTS safe/shs/index.htm Last Updated: 30-Sep-2005 http://www.nps.gov/history/history/online_books/safe/index.htm[7/2/2012 3:03:56 PM] Santa Fe National Historic Trail: Special History Study (Table of Contents) SANTA FE Special History Study TABLE OF CONTENTS COVER LIST OF FIGURES INTRODUCTION CHAPTER I - Isolation and Dependency CHAPTER II - Poverty and Neglect CHAPTER III - Going Down the Royal Road CHAPTER IV - Contraband and the Law CHAPTER V - New Mexican Merchants and Mercantile Capitalism CHAPTER VI - Felipe Chavez CHAPTER VII - Other Leading Merchant Families CONCLUSION RECOMMENDATIONS FOR FURTHER STUDY BIBLIOGRAPHY GLOSSARY APPENDIX I APPENDIX II APPENDIX III APPENDIX IV LIST OF ILLUSTRATIONS Figure 1. "Loading Up." J. Gregg marveled at the dexterity and skill with which hispanos harnessed and adjusted packs of merchandise Figure 2. The Santa Fe Trade: An International Trade Network Figure 3. The Santa Fe Trail: Part of an International Trade Network http://www.nps.gov/history/history/online_books/safe/shst.htm[7/2/2012 3:04:00 PM] Santa Fe National Historic Trail: Special History Study (Table of Contents) Figure 4. Pedro Vial pioneered a route that closely resembled the one Santa Fe Traders would follow in the next century Figure 5. -

I Identification and Characterization of Martian Acid-Sulfate Hydrothermal
Identification and Characterization of Martian Acid-Sulfate Hydrothermal Alteration: An Investigation of Instrumentation Techniques and Geochemical Processes Through Laboratory Experiments and Terrestrial Analog Studies by Sarah Rose Black B.A., State University of New York at Buffalo, 2004 M.S., State University of New York at Buffalo, 2006 A thesis submitted to the Faculty of the Graduate School of the University of Colorado in partial fulfillment of the requirement for the degree of Doctor of Philosophy Department of Geological Sciences 2018 i This thesis entitled: Identification and Characterization of Martian Acid-Sulfate Hydrothermal Alteration: An Investigation of Instrumentation Techniques and Geochemical Processes Through Laboratory Experiments and Terrestrial Analog Studies written by Sarah Rose Black has been approved for the Department of Geological Sciences ______________________________________ Dr. Brian M. Hynek ______________________________________ Dr. Alexis Templeton ______________________________________ Dr. Stephen Mojzsis ______________________________________ Dr. Thomas McCollom ______________________________________ Dr. Raina Gough Date: _________________________ The final copy of this thesis has been examined by the signatories, and we find that both the content and the form meet acceptable presentation standards of scholarly work in the above mentioned discipline. ii Black, Sarah Rose (Ph.D., Geological Sciences) Identification and Characterization of Martian Acid-Sulfate Hydrothermal Alteration: An Investigation -

Appendix I Lunar and Martian Nomenclature
APPENDIX I LUNAR AND MARTIAN NOMENCLATURE LUNAR AND MARTIAN NOMENCLATURE A large number of names of craters and other features on the Moon and Mars, were accepted by the IAU General Assemblies X (Moscow, 1958), XI (Berkeley, 1961), XII (Hamburg, 1964), XIV (Brighton, 1970), and XV (Sydney, 1973). The names were suggested by the appropriate IAU Commissions (16 and 17). In particular the Lunar names accepted at the XIVth and XVth General Assemblies were recommended by the 'Working Group on Lunar Nomenclature' under the Chairmanship of Dr D. H. Menzel. The Martian names were suggested by the 'Working Group on Martian Nomenclature' under the Chairmanship of Dr G. de Vaucouleurs. At the XVth General Assembly a new 'Working Group on Planetary System Nomenclature' was formed (Chairman: Dr P. M. Millman) comprising various Task Groups, one for each particular subject. For further references see: [AU Trans. X, 259-263, 1960; XIB, 236-238, 1962; Xlffi, 203-204, 1966; xnffi, 99-105, 1968; XIVB, 63, 129, 139, 1971; Space Sci. Rev. 12, 136-186, 1971. Because at the recent General Assemblies some small changes, or corrections, were made, the complete list of Lunar and Martian Topographic Features is published here. Table 1 Lunar Craters Abbe 58S,174E Balboa 19N,83W Abbot 6N,55E Baldet 54S, 151W Abel 34S,85E Balmer 20S,70E Abul Wafa 2N,ll7E Banachiewicz 5N,80E Adams 32S,69E Banting 26N,16E Aitken 17S,173E Barbier 248, 158E AI-Biruni 18N,93E Barnard 30S,86E Alden 24S, lllE Barringer 29S,151W Aldrin I.4N,22.1E Bartels 24N,90W Alekhin 68S,131W Becquerei -

Estimates of Some Physical/Mechanical Properties
U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY ESTIMATES OF SOME PHYSICAL/MECHANICAL PROPERTIES OF MARTIAN ROCKS AND SOILLIKE MATERIALS H. J. MOORE1 Open-File Report 91-568 Prepared under NASA Contract W-15,814 This report is preliminary and has not been reviewed for conformity with the U.S. Geological Survey editorial standards (or with the North American Stratigraphic Code). Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. 1 U.S. Geological Survey, 345 Middlefield Road, Menlo Park, CA 94025 1991 ESTIMATES OF SOME PHYSICAL/MECHANICAL PROPERTIES OF MARTIAN ROCKS AND SOILLIKE MATERIALS H. J. Moore U.S. Geological Survey 345 Middlefield Road Menlo Park, CA 94025 ABSTRACT This paper addresses questions on Martian surface materials (exclusive of the polar regions) that were posed at a Sand and Dust Workshop in February, 1991. Materials thought to be most likely encountered on Mars are dust, soillike materials, sand sheets and dunes, and bedrock and rock fragments. In response to a need expressed by engineers attending the workshop, selected physical/mechanical properties of these materials are discussed: grain size, hardness, and shape; density; friction angle; cohesion; compressibility; adhesion; thermal inertia; and dielectric constant and loss tangent. Numerical values of these properties are presented for each material in tables. Some properties, such as bearing strength, are only briefly discussed because they are derived from other properties. Additionally, other factors affecting the surface materials and their responses to loads, such as surface gravity, are discussed. In conclusion, four specific questions posed at the workshop are addressed. -

Executive-Order-No.-422-Re.-Travel-Policies-And-Procedures.Pdf
Republic o-r Palau 0/iice oi die <:Presi"iJent P.O. Box 6051, Palau, PW 96940 Tommy E Remengesau, jr. Tel. (6801 767-2403/2828 !lJ~ Fax. (680)767-2424/1662 Email: rop. [email protected] EXECUTIVE ORDER NO. 422 Revising the Republic ofPalau Travel Policies and Procedures for the Executive Branch ofthe National Government WHEREAS, it is necessary to establish and maintain travel policies and procedures for the Executive Branch of the National Government in a single, accessible, and comprehensive document; and WHEREAS, such travel policies and procedures need to reflect current management practices, desires and terminologies; and WHEREAS, it is thus necessary to periodically update and revise the travel policies and procedures to reflect the changing needs and realities of managing travel funded by the Executive Branch; and WHEREAS, the Executive Branch's comprehensive travel policies and procedures were last revised in February of 2017 and require adjustments to account for circumstances that have changed during this time, including an adjustment of the applicable per diem rates to accurately reflect the present cost of travel; NOW, THEREFORE, by virtue of the authority vested in me as President of the Republic of Palau, and pursuant to the Constitution and laws of the Republic of Palau, I do hereby order that the document entitled "Republic of Palau Travel Policies and Procedures for the Executive Branch of the National Government," as revised on April 2019, be put into effect as of the date of this Executive Order. This Executive Order supersedes all previous orders and directives regarding travel policies and procedures, including specifically Executive Order No. -
1 Ccr 101-1 State Fiscal Rules 2 (Pdf)
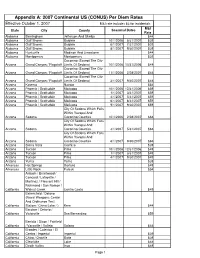
Appendix A: 2007 Continental US (CONUS) Per Diem Rates Effective October 1, 2007 M&I rate includes $3 for incidentals M&I State City County Seasonal Dates Rate Alabama Birmingham Jefferson And Shelby $44 Alabama Gulf Shores Baldwin 10/1/2006 5/31/2007 $39 Alabama Gulf Shores Baldwin 6/1/2007 7/31/2007 $39 Alabama Gulf Shores Baldwin 8/1/2007 9/30/2007 $39 Alabama Huntsville Madison And Limestone $44 Alabama Montgomery Montgomery $39 Coconino (Except The City Arizona Grand Canyon / Flagstaff Limits Of Sedona) 10/1/2006 10/31/2006 $44 Coconino (Except The City Arizona Grand Canyon / Flagstaff Limits Of Sedona) 11/1/2006 2/28/2007 $44 Coconino (Except The City Arizona Grand Canyon / Flagstaff Limits Of Sedona) 3/1/2007 9/30/2007 $44 Arizona Kayenta Navajo $54 Arizona Phoenix / Scottsdale Maricopa 10/1/2006 12/31/2006 $59 Arizona Phoenix / Scottsdale Maricopa 1/1/2007 3/31/2007 $59 Arizona Phoenix / Scottsdale Maricopa 4/1/2007 5/31/2007 $59 Arizona Phoenix / Scottsdale Maricopa 6/1/2007 8/31/2007 $59 Arizona Phoenix / Scottsdale Maricopa 9/1/2007 9/30/2007 $59 City Of Sedona Which Falls Within Yavapai And Arizona Sedona Coconino Counties 10/1/2006 2/28/2007 $64 City Of Sedona Which Falls Within Yavapai And Arizona Sedona Coconino Counties 3/1/2007 5/31/2007 $64 City Of Sedona Which Falls Within Yavapai And Arizona Sedona Coconino Counties 6/1/2007 9/30/2007 $64 Arizona Sierra Vista Cochise $39 Arizona Tucson Pima 10/1/2006 12/31/2006 $49 Arizona Tucson Pima 1/1/2007 3/31/2007 $49 Arizona Tucson Pima 4/1/2007 9/30/2007 $49 Arizona Yuma Yuma -

The Physics of Wind-Blown Sand and Dust
The physics of wind-blown sand and dust Jasper F. Kok1, Eric J. R. Parteli2,3, Timothy I. Michaels4, and Diana Bou Karam5 1Department of Earth and Atmospheric Sciences, Cornell University, Ithaca, NY, USA 2Departamento de Física, Universidade Federal do Ceará, Fortaleza, Ceará, Brazil 3Institute for Multiscale Simulation, Universität Erlangen-Nürnberg, Erlangen, Germany 4Southwest Research Institute, Boulder, CO USA 5LATMOS, IPSL, Université Pierre et Marie Curie, CNRS, Paris, France Email: [email protected] Abstract. The transport of sand and dust by wind is a potent erosional force, creates sand dunes and ripples, and loads the atmosphere with suspended dust aerosols. This article presents an extensive review of the physics of wind-blown sand and dust on Earth and Mars. Specifically, we review the physics of aeolian saltation, the formation and development of sand dunes and ripples, the physics of dust aerosol emission, the weather phenomena that trigger dust storms, and the lifting of dust by dust devils and other small- scale vortices. We also discuss the physics of wind-blown sand and dune formation on Venus and Titan. PACS: 47.55.Kf, 92.60.Mt, 92.40.Gc, 45.70.Qj, 45.70.Mg, 45.70.-n, 96.30.Gc, 96.30.Ea, 96.30.nd Journal Reference: Kok J F, Parteli E J R, Michaels T I and Bou Karam D 2012 The physics of wind- blown sand and dust Rep. Prog. Phys. 75 106901. 1 Table of Contents 1. Introduction .......................................................................................................................................................... 4 1.1 Modes of wind-blown particle transport ...................................................................................................... 4 1.2 Importance of wind-blown sand and dust to the Earth and planetary sciences ........................................... -

Buscapronta Base De Dados Junho/2010
BUSCAPRONTA WWW.BUSCAPRONTA.COM BASE DE DADOS JUNHO/2010 9.264.260 SOBRENOMES / ENDEREÇOS PESQUISADOS SITES E LINKS A 145147 B 1347538 C 495817 D 276690 E 17216 F 177217 G 105102 H 61772 I 4082 J 29600 K 655085 L 42311 M 550085 N 540620 O 4793 P 117441 Q 226 R 1716485 S 1089982 T 71176 U 35625 V 47393 W 1681776 X / Y 1490 Z 49591 ATUALIZAÇÕES E PÁGINAS OFF LINE PODEM ALTERAR (PARA MAIS OU PARA MENOS) OS PRESENTES NÚMEROS EM ATÉ 5% A 8% ATENÇÃO Os números apresentados à direita de alguns sobrenomes indicam o número de sites e links correspondentes. Links podem se referir a um ou mais indivíduos. Sobrenomes sem numeração à direita se referem a famílias, indivíduo ou grupo de indivíduos. Relação de sobrenomes apresentada em 3 segmentos distintos (um não substitui o outro quando apresentar o mesmo sobrenome). Eventualmente, poderá ocorrer superposição de informação sobre um mesmo indivíduo, em endereços Internet diferentes. Este arquivo geral não substitui os demais arquivos apresentados no site BUSCAPRONTA. Para facilitar sua pesquisa utilize a ferramenta EDITAR/LOCALIZAR so WORD. BUSCAPRONTA não reproduz dados genealógicos. È necessário acessar os sites e links correspondentes. As informações contidas nos sites e links são de única e exclusiva responsabilidade de seus respectivos editores. O Banco de Dados do BUSCAPRONTA é complementado diariamente com novos dados eventualmente não apresentados neste resumo geral. Se você não encontrou aqui todos os dados de que necessita para sua pesquisa entre em contato e informe sobre os sobrenomes -

Mars Pathfinder Landing Site Workshop Ii: Characteristics of the Ares Vallis Region and Field Trips in the Channeled Scabland, Washington
/, NASA-CR-200508 L / MARS PATHFINDER LANDING SITE WORKSHOP II: CHARACTERISTICS OF THE ARES VALLIS REGION AND FIELD TRIPS IN THE CHANNELED SCABLAND, WASHINGTON LPI Technical Report Number 95-01, Part 1 Lunar and Planetary Institute 3600 Bay Area Boulevard Houston TX 77058-1113 LPI/TR--95-01, Part 1 "lp MARS PATHFINDER LANDING SITE WORKSHOP II: CHARACTERISTICS OF THE ARES VALLIS REGION AND FIELD TRIPS IN THE CHANNELED SCABLAND, WASHINGTON Edited by M. P. Golombek, K. S. Edgett, and J. W. Rice Jr. Held at Spokane, Washington September 24-30, 1995 Sponsored by Arizona State University Jet Propulsion Laboratory Lunar and Planetary Institute National Aeronautics and Space Administration Lunar and Planetary Institute 3600 Bay Area Boulevard Houston TX 77058-1113 LPI Technical Report Number 95-01, Part 1 LPI/TR--95-01, Part 1 Compiled in 1995 by LUNAR AND PLANETARY INSTITUTE The Institute is operated by the University Space Research Association under Contract No. NASW-4574 with the National Aeronautics and Space Administration. Material in this volume may he copied without restraint for library, abstract service, education, or personal research purposes; however, republication of any paper or portion thereof requires the written permission of the authors as well as the appropriate acknowledgment of this publication. This report may he cited as Golomhek M. P., Edger K. S., and Rice J. W. Jr., eds. ( 1992)Mars Pathfinder Landing Site Workshop 11: Characteristics of the Ares Vallis Region and Field Trips to the Channeled Scabland, Washington. LPI Tech. Rpt. 95-01, Part 1, Lunar and Planetary Institute, Houston. 63 pp.