Habitat Suitability for Juvenile Flatfish of the Inner Danish Waters Section for Ecosystem Based Marine Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
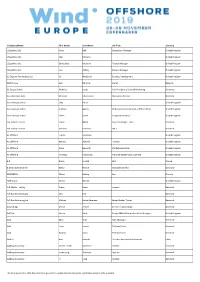
Company Name First Name Last Name Job Title Country
Company Name First Name Last Name Job Title Country 1StopWind Ltd Arran Bell Operations Manager United Kingdom 1StopWind Ltd. Alan Mckerns United Kingdom 1StopWind Ltd. Bernadette McAulay Finance Manager United Kingdom 1StopWind Ltd. Joel Telling General Manager United Kingdom 23 Degrees Renewables Ltd Ed Woodrow Business Development United Kingdom 24SEA bvba Gert De Sitter Owner Belgium 3S Europe GmbH Matthias Lamp Vice President of Sales & Marketing Germany 3sun Denmark ApS Christian Christensen Operations Director Denmark 3sun Group Limited Jody Potter United Kingdom 3sun Group Limited Graham Hacon VP Business Development, Offshore Wind United Kingdom 3sun Group Limited Sherri Smith Company Secretary United Kingdom 3W Industri Service Simon Øland Project manager - sales Denmark 3W Industri Service Kenneth Pedersen IWI-S Denmark 4C Offshore Lauren Anderson United Kingdom 4C Offshore Richard Aukland Director United Kingdom 4C Offshore Rosie Haworth Market Researcher United Kingdom 4C Offshore Vincenzo Poidomani Principal Geotechnical Engineer United Kingdom 8.2 Bruno ALLAIN CEO France 8.2 Monitoring GmbH Bernd Höring Managing director Germany 920338402 Ellinor Meling Ceo Norway A&P Group Emma Harrick United Kingdom A.P. Møller Holding Simon Ibsen Investor Denmark A/S Dan-Bunkering Ltd. Jens Kirk Denmark A/S Dan-Bunkering Ltd. Michael Brunø-Sørensen Senior Bunker Trader Denmark A1wind Aps Martin Jensen Director / A1wind Aps Denmark AAF Ltd Steven Brett Europe MFAS Aftermarket Sales Manager United Kingdom AAG Allan Tarp Sales Manager Denmark -

Hesselø Bugt Miljøvurderingsrapport (VVM)
Gribskov Kommune på vegne af Nordkystens Fremtid Råstofindvinding i ansøgningsområde A – Hesselø Bugt Miljøvurderingsrapport (VVM) Rekvirent Gribskov Kommune på vegne af Nordkystens Fremtid Kontaktperson: Caroline Bald (e-mail:[email protected]) Rådgiver WSP Danmark før Orbicon Linné Alle 2 2630 Taastrup Projektnummer 3621700085 Projektleder Jan F. Nicolaisen Udarbejdet af Louise K. Poulsen, Danni J. Jensen, Morten Warnick Stæhr, Morten Hjort, Sanne Kjellerup, Erik Mandrup Jacobsen, Rie B. E. Jensen Kort Morten Warnick Stæhr, Danni J. Jensen, Rie B. E. Jensen Kvalitetssikring Jan F. Nicolaisen Revisionsnr. 02 Godkendt af Lea Bjerre Schmidt Udgivet 19-05-2021 INDHOLDSFORTEGNELSE 1. IKKE-TEKNISK RESUMÉ.................................................................................................... 9 2. INDLEDNING..................................................................................................................... 12 2.1. Baggrund ................................................................................................................. 12 2.2. Læsevejledning ........................................................................................................ 15 3. PROJEKTBESKRIVELSE ................................................................................................. 16 3.1. Områdebeskrivelse .................................................................................................. 16 3.2. Indvindingen ............................................................................................................ -

Coastal Meadow Management
coastal meadow management Best Practice Guidelines The experiences of LIFE-Nature project “Boreal Baltic Coastal Meadow Preservation in Estonia” LIFE00NAT/EE/7083 coastal meadow management Best Practice Guidelines MATSALU ESTONIAN MINISTRY DANCEE NATIONAL PARK OF ENVIRONMENT The experiences of LIFE-Nature project “Boreal Baltic Coastal Meadow Preservation in Estonia” LIFE00NAT/EE/7083 Compiled by Riinu Rannap, Lars Briggs, Kaja Lotman, Ilona Lepik, Voldemar Rannap Translated by Pirkko Põdra Photos Arne Ader, Lars Briggs, Fred Jüssi, Tiit Kaljuste, Mati Kose, Ilona Lepik, Kaja Lotman, Riinu Rannap, Voldemar Rannap, Merike Tamm, Ülle Tamm Drawings Elen Apsalon Layout Eerik Keerend This book has been printed on CyclusPrint recycled paper Ministry of the Environment of the Republic of Estonia Tallinn 2004 ISBN 9985-881-26-5 LIFE-Nature Project By way of introduction. Riinu Rannap, Voldemar Rannap 4 content Management Coastal meadow as a habitat. Kaja Lotman, Ilona Lepik 8 Amphibians • birds • plants Boreal Baltic coastal meadow management for Bufo calamita. Riinu Rannap 26 Restoration of breeding sites for threatened toads on coastal meadows. Lars Briggs 34 Suitable habitat management for Danish bird populations. Ole Thorup 44 Changes of bird communities in relation to management of coastal meadows in Estonia. Andres Kuresoo, Eve Mägi 52 Coastal meadow management from a botanist’s point of view. Tiit Kaljuste 62 Monitoring the Wild gladiolus (Gladiolus imbricatus) population under different meadow management regimes. Marika Kose, Mari Moora 70 Experiences The socio-economic aspect of coastal meadow management: the Matsalu example. Kaja Lotman 72 Managing meadows or managing people? Coastal meadow restoration and management in the Häädemeeste region. -

Dansk Fyrliste 2020 1 2 3 4 5 6 7 8 Dansk Nr./ Navn/ Bredde/ Fyrkarakter/ Flamme- Lysevne Fyrudseende/ Yderligere Oplysninger Int
Fyr · Tågesignaler · R acon · AIS · DGPS Dansk Fyrliste Danmark · Færøerne · Grønland 38. udgave 2020 Titel: Dansk Fyrliste, 38. udgave. Forsidefoto: Mykines Hólmur (Myggenæs) Fyr Fyr nr. 6890 (L4460) Bagsidefoto: Skagen Fyr Fyr nr. 330 (C0002) Fotograf: Lars Schmidt, Schmidt Photography © Søfartsstyrelsen 2021 INDHOLDSFORTEGNELSE 1. Forord ......................................................................................... 2 4. AIS-afmærkning ..................................................................... 342 1.1 Anvendte forkortelser ....................................................................... 2 4.1 Forklaring til oplysninger om AIS................................................... 342 2. Fyr og tågesignaler ..................................................................... 3 4.2 Fortegnelse over AIS-afmærkninger .............................................. 343 2.1 Forklaring til oplysninger om fyr og tågesignaler ................................ 3 5. DGPS-referencestationer ........................................................ 347 2.2 Anvendte fyrkarakterer ..................................................................... 4 5.1 Forklaring til oplysninger om DGPS .............................................. 347 2.3 Fyrs optiske synsvidde ved varierende sigtbarhed .............................. 5 5.2 Fortegnelse over DGPS-referencestationer ..................................... 348 2.4 Geografisk synsvidde ved varierende flamme- og øjenhøjde ............... 6 2.5 Fortegnelse over fyr og tågesignaler: -

IV. Report on Birds in Danmark, R886. Garis
--------------------------------------------------------------------------------------1 368 V. v. Tschusi und K. v, Dalla-Torre. 4· Hinmdo rustica und Hirundo urbica, Gal!inula por- rana. Mit Bericht des Leuchtthurm - Assistenten von Zaglava auf Cherso vom g. April 1885 erhielt ich 3 3 todte Rauch· schwalben, eine Hausschwalbe und eine Gallinula porr_ana. 5. Alauda arvensis, Dandalus rubecula und Sturn us vul- IV. Report on Birds in Danmark, r886. garis. Mit Bericht vom Leuchtthurm-Assistenten A. 0 mer o auf Compiled by Punta d' Ostro vom rg. October r885 erhielt ich zehn Alauda arvensis, einen Dandalus rubecula und einen Strlrnus vulgaris, Oluf Winge. welche Vogel am Zuge in der Nacht vom 18. auf r 9· October, (With a Map-Plate I.) gegen 3 Uhr morgens, bei leichtem Regen am Leuchtthurme anstiessen. Observations have been communicated by: A.: H. Arctander, physician, Storehedinge, Stevns, 6. Sturnus vulgaris. Mit Bericht des Assistenten von Gollo~ Sjalland. Observations in Stevns. metto . bei Grado ddo. 2. Mi.irz r886 flogen urn Mitternacht F.: A. H. Faber, cand. pharm. Notes from Viborg r Uhr an die Laterne zwei Staare an, deren Schnabel gebogen and surroundings, within three or four (Danish) miles from und gespalten waren. the town. Species from Viborg 88. - Also some notes from Hafenagentie Grado, 2, Marz r886. visits to Mors, where the observer lived during r 884 and 188S*). Der J\Iandatar flir Istrien: Hs.: P. Hersch end, possessor of Herschendsga ve, Dr. L. K Moser. S. E. of Skanderborg So, Jylland. K.: Th. N. Krabbe, stud. me d. Chiefly observations from the island of Amager, especially the coasts and the northern part close to Kjobenhavn, with the fortification territory. -

Executive Order No. 680 of 18 July 2003, Amending Executive Order No
Division for Ocean Afhirs and the Law of the Sea OSficc. oi' Legal Affairs Law of the Sea United Nations New York, 2004 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status \>fany country, territory. city or area or of its authorities, or concerning the delimitation of its frontiers 0:- boundaries. Furthermore, publication in the Bulletin of information cvncerning developments relating to the law of thc sea emanating from actions and decisions taken by the States does not imply recognition by the United Nations of the validity of the actions anti decisions in question. IF ANY MATERIAL CONTAINED IN 7 YE BL'LLETIN IS KFPRODC'CED IN PART OR IN WHOLE, DUE AC'KNOWL.EDGEMENT SHOl !LD BE G1VF.N Copyright (' IJnitcd Nations. 1904 CONTENTS Page I. UNITED NATIONS CONVENTION ON THE LAW OF THE SEA ........................................ 1 Status of the United Nations Convention on the Law of the Sea, of the Agreement relating to the implementation of Part XI of the Convention and of the Agreement for the implementation of the provisions of the Convention relating to the conservation and management of straddling fish stocks and highly migratory fish stocks .............................................................................................................. 1 1. Table recapitulating the status of the Convention and of the related Agreements, as at 30 November 2003.......................................................................................................... 1 2. Chronological lists of ratifications of, accessions and successions to the Convention and the related Agreements, as at 30 November 2003............................................................................ 12 (a) The Convention ................................................................................................................ -

De Danske Fuglestationer: Fra Jordsand Til Christiansø
De danske fuglestationer: fra Jordsand til Christiansø PETER LYNGS Når man som gammel fuglestationsmand skal for- natur og fugle, som begyndte at spire i starten af tælle om de danske fuglestationers historie, kan det 1900-tallet. DOFs oprettelse i 1906 var et udtryk ikke undgås, at man – og dermed denne beretning for denne interesse, samt for en stigende bekym- – er farvet af den tid og de stationer, man har fær- ring for den måde vores natur blev forvaltet på. dedes på. Denne fortælling bliver derfor præget af Disse tanker, om end stadig 'få og små', deltes af personlige valg, og andre ville have valgt anderle- andre, og på den politiske side medførte de bl.a. des. Fortællingen bliver også summarisk, for hver oprettelsen af Naturfredningsrådet i 1917. I 1936 stations historie kunne være blevet til en novelle, blev der, med hjemmel den nye reservatlov, opret- endog en roman. tet en række naturvidenskabelige reservater, bl.a. Jeg trådte mine ungdomssko i Blåvand og drev Tipperne, Vorsø og Græsholmen ved Christiansø. som flere andre blåvandobservatører mod øst til Administrationen af disse reservater kom ind un- Christiansø. Andre foretrak Tippernes udstrakte der Naturfredningsrådet, hvilket muliggjorde et enge, Vejlernes sumpe, Kattegats småøer eller målrettet videnskabeligt arbejde og lagde grunden Sjællands kyster. For nogen blev fuglestationerne for de første danske fuglestationer. jordens salt, for andre en episode i deres liv. Men Årene efter Anden Verdenskrig førte en stigende alle oplevede den glæde, det giver at arbejde in- velstand med sig, og en lang række mennesker fra tenst med vilde fugle, ikke mindst sammen med de store fødselsårgange i 1940erne og 1950erne ligesindede, og for langt de fleste har det været en fik stor interesse for fugle og natur. -

Changes in Danish Bird 1966-75 Numbers of Migrants Observatories
Changes in numbers of migrants ringed at Danish bird observatories during the years 1966-75 FINN DALBERG PETERSEN (Med et dansk resume: Ændringer i antallet af ringmærkede trækfugle på nogle danske fuglestationer i perioden 1966 - 75) INTRODUCTION Christians Ø breed in Sweden, Finland, and partly westernmost Russia. In the autumns of 1968 - 69 - 70 a constant In the years of 1966 - 1971 ringing was trapping of birds was carried out at Bodensee carried out at Hesselø during the following in southernmost Germany (Berthold 1972). periods: 1) April 16 -June 22 1966, 2) April During these three years nearly all species 27-June 6 1967, 3) April 8-May 31 1969, showed a significant decline in the number 4) April 25 - June 2 1970, and 5) April 3 - ringed, and Berthold considers this decrease May 25 1971 (Rabøl 196 7, 1969, Tønder & as indicative of "exceptionally high or Rabøl 1972, Møller & Petersen 1973 ). The longterm declines 11 of the breeding birds were trapped in nets, which were populations in the recruitment areas of the distributed all over the island in all kinds of Bodensee migrants. The recruitment area is vegetation. The nets were open every day thought to be Middle Europe north of Boden from dawn to the middle of the afternoon, and see. Further reports on declines since 1968, numbered 15 - 20 in 1966 - 67, 30 in 1969, especially of Redstart Phoenicurus phoeni and 22 - 25 in 1970 - 71. In order to correct curus, Sedge Warbler Acrocephalus schoeno the influence of the different numbers of nets baenus, and Whitethroat Sylvia communis, on the numbers of ringed birds I have divided have been published (Glue 1970, Batten the numbers of ringings by 3 ( 1966 - 67), 5 1971, Berthold 1973, 1974,.Wink 1974, Win (1969), and 4 (1970 - 71) (Tab le 1). -

Executive Order No 242 of 21 April 1999
Page 1 Executive Order No 242 of 21 April 1999 Executive Order concerning the Delimitation of Denmark’s Territorial Sea In pursuance of section 2 (3) and (4) of Act No. 200 of 7 April 1999 on the Delimitation of the Territorial Sea, the following provisions are hereby laid down: 1. The external territorial waters cover those areas of the sea which landward are delimited by the baselines mentioned in section 2 and seaward by lines drawn in such a manner that the distance from every point of those lines to the nearest point of the baselines is 12 nautical miles (22,224m), cf., however, sections 3-5. The boundary lines are depicted in the chart enclosed with this Executive Order. 2. The baselines on which the measuring of the external territorial waters is based in pursuance of section 1 shall be the coastal line and straight lines as indicated below between the following points: Jutland-Fenen 1. 55º04’06”.3N 8º23’17”.8E Danish-German maritime boundary from there a straight line to 2. 55º12’ 7".6N 8º24’09”.4E Romo W from there a straight line to 3. 55º19’44”.6N 8º24’52”.4E Galgerev (Fanø S) from there a straight line to 4. 55º26’37”.6N 8º18’43”.4E Soren Jessens Sand from there a straight line to National legislation - DOALOS/OLA - United Nations asdf Page 2 5. 55º28’23”.6N 8º17’00”.4E Skallingen W from there the coastal line to 6. 55º59’55”.5N 8º06’51”.7E Hvide Sande S from there a straight line to 7. -

Danmarks Ynglebestand Af Skarver 2017
DANMARKS YNGLEBESTAND AF SKARVER 2017 Teknisk rapport fra DCE – Nationalt Center for Miljø og Energi nr. 103 2017 AARHUS AU UNIVERSITET DCE – NATIONALT CENTER FOR MILJØ OG ENERGI [Tom side] DANMARKS YNGLEBESTAND AF SKARVER 2017 Teknisk rapport fra DCE – Nationalt Center for Miljø og Energi nr. 103 2017 Thomas Bregnballe1 Max Nitschke2 1 Aarhus Universitet, Institut for Bioscience 2 Pro Insecta AARHUS AU UNIVERSITET DCE – NATIONALT CENTER FOR MILJØ OG ENERGI Datablad Serietitel og nummer: Teknisk rapport fra DCE - Nationalt Center for Miljø og Energi nr. 103 Titel: Danmarks ynglebestand af skarver i 2017 Forfattere: Thomas Bregnballe1 & Max Nitschke2 Institutioner: 1Aarhus Universitet, Institut for Bioscience og 2Pro Insecta Udgiver: Aarhus Universitet, DCE – Nationalt Center for Miljø og Energi © URL: http://dce.au.dk Udgivelsesår: August 2017 Redaktion afsluttet: August 2017 Faglig kommentering: Kevin K. Clausen Kvalitetssikring, DCE: Jesper R. Fredshavn Finansiel støtte: Miljøstyrelsen Bedes citeret: Bregnballe, T. & Nitschke, M. 2017. Danmarks ynglebestand af skarver i 2017. Aarhus Universitet, DCE – Nationalt Center for Miljø og Energi, 40 s. - Teknisk rapport fra DCE - Nationalt Center for Miljø og Energi nr. 103 http://dce2.au.dk/pub/TR103.pdf Gengivelse tilladt med tydelig kildeangivelse Sammenfatning: Ved årets optælling af ynglende skarver i Danmark blev der registreret 33.171 ynglepar. Yngleantallet var således steget med 5-8 % i forhold til de forudgående 3 år, men der var fortsat omkring 6.000 færre ynglepar end i årene 1993-2006. I 2017 var der 79 kolonier, hvilket er 4 færre end i 2016. I forhold til 2016 var der i 2017 tilbagegang i Limfjorden og i det nordlige Sjælland, hvorimod der var fremgang i de vestjyske fjorde, i regionen omfattende Lillebælt og Det Sydfynske Øhav samt i Smålandsfarvandet. -

Forskningsberetning 2000
1$7,21$/086((76 )2561,1* 1DWLRQDOPXVHHW 0DM 1 )RURUG Forskningsberetning 2000 er en afrapportering af forskningsindsatsen på Nationalmuseets ti nuværende forskningsområder, forskningssekretariatets og Bibliotekstjenestens funktioner. I udformningen af beretningen er der taget hensyn til den rådgivning som Nationalmuseets Eksterne Forskningsudvalg (NEF) har fremført ved behandlingen af ”Nationalmuseets Forskning” (Forsk- ningsberetning 1999 og Forskningsplan 2000-2003). Dette afspejler sig i en række ændringer i struktur og indhold i Forskningsberetning 2000. Hovedændringen i forhold til den tidligere beretning er, at museets ti forskningsområder udgør hovedinddelingen. Dermed bliver det enkelte forskningsområdes aktiviteter beskrevet samlet og ikke som tidligere under to forskellige principper, forskningsområde og organisatoriske tilhørsforhold. Det er desuden tilstræbt at medtage beskrivelser af de enkelte forskningsområders basisaktiviteter eller aktiviteter som ikke er medtaget i projektkataloget. Som noget nyt er der tilføjet et afsnit som indeholder en række mindre artikler med interessante og nye problemstillinger inden for en række af forskningsområderne. Beretningen indeholder tre afsnit: I. Nationalmuseets forskning, generelt, II. Glimt fra forskningsområderne og III. Forskningsområdernes aktiviteter. Desuden som bilag et samlet Projektkatalog. )RUVNQLQJVVHNUHWDULDWHW 2 ,1'+2/'6)257(*1(/6( )2525' , 1$7,21$/086((76 )2561,1*................................................................. p.1 1.1. Målsætning ......................................................................................................... -

Hver Ø – Sin Charme
Geografisk Orientering Tema: Danske småøer Tidsskrift for Geografforbundet April 2009 · 39. årgang · Nr. 2 Geografisk Orientering Indhold Geografforbundets medlemsblad Medlemskontingent for 2008-2009: Almindeligt medlemskab: 275 kr. *Velkommen til de danske småøer! ....................................... 68 Familie (par): 350 kr. Studerende 125 kr. Claus Jensen Institutioner, skoler: 450 kr. *Naturen på danske småøer ................................................... 74 Henvendelse om medlemskab/abonnement m.v.: Geografforlaget, Filosofgangen 24, 5000 Odense C Poul Henrik Harritz 63 44 16 83, Fax 63 44 16 97 e-mail: [email protected] *Danske øers dannelse og landskab ....................................... 81 Hjemmeside: www.geografforbundet.dk Henrik Nørregaard Redaktion Ansvarshavende redaktør og annoncetegning: Månedens link......................................................................... 87 Mette Starch Truelsen, 49 21 60 21 P. W. Tegners Vej 91, 3070 Snekkersten *Øproblemer i Danmark ......................................................... 88 e-mail: [email protected] Niels Ulrik Kampmann Hansen Anmelderredaktør: Maja Enghave Kristensen, 35 26 12 37 *Fur mellem fortid og fremtid ............................................. 100 Gamlevældevej 42, 3760 Gudhjem John Brinch Bertelsen Søren Pilgaard Kristensen, 50 92 12 71 Henning Strand, 33 24 07 37 Leif Tang Lassen, 48 30 00 95 *Hvor kan man læse om de danske øer? .............................. 110 Anne Dorte Hjernø (gym.), 44 99 65 21 Niels Ulrik Kampmann Hansen