Food Web E.2. Electronic Appendix
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Bibliography of Scientific Information on Fraser River Basin Environmental Quality
--- . ENVIRONMENT CANADA — b- A BIBLIOGRAPHY OF SCIENTIFIC INFORMATION ON FRASER RIVER BASIN ENVIRONMENTAL QUALITY . 1994 Supplement e Prepared on contract by: Heidi Missler . 3870 West 11th Avenue Vancouver, B.C. V6R 2K9 k ENVIRONMENTAL CONSERVATION BRANCH PACIFIC AND YUKON REGION NORTH VANCOUVER, B.C. L- ,- June 1994 DOE FRAP 1994-11 *- \- i — --- ABSTRACT -. -. This bibliography is the third in a series of continuing reference books on the Fraser River watershed. It includes 920 references of scientific information on the environmental I quality of the Fraser River basin and is both an update and an extension of the preceding -. bibliography printed in 1992. ,= 1- ,- . 1- 1- !- 1 - — ii — RESUME — La presente bibliographic est la troiseme clans une serie continue portant sur le bassin du fleuve Fraser. Elle comprend 920 citations scientifiques traitant de la qualite de l’environnement clans le bassin du fleuve Fraser, et elle constitue une mise a jour de la bibliographic precedence, publiee en 1992. — — — ---- — —. .— — — ,- .— ... 111 L TABLE OF CONTENTS Page Abstract ‘ i Resume ii Introduction iv References Cited v Acknowledgements vi Figure: 1. Fraser River Watershed Divisions , vii ... Tables: 1. Reference Locations Vlll 2. Geographic Location Keywords ix 3. Physical Environment Keywords x 4. Contamination Kefiords xi, 5. Water Quality Keywords xii . ... 6. Natural Resources Keywords Xlll 7. Biota Keywords xiv 8. General Keywords xv Section One: Author Index Section Two: Title Index \ 117 ( L iv INTRODUCTION This bibliography is the third in a series of continuing reference books on the Fraser River watershed. With its 920 references of scientific information on the environmental quality of the , -. -

A Backup Plan
FWCP NEWS fwcp.ca BC HYDRO | PROVINCE OF B.C. | FISHERIES AND OCEANS CANADA There is a real risk of provincial extirpation, making this captive assurance program so essential for the northern leopard frog. Photo courtesy of Doug Adama. Check out our new look! The Fish and Wildlife Compensation Program has redesigned their newsletter to share success stories from across B.C. A BACKUP PLAN The Update newsletter from the Columbia region and the Natureline newsletter from the Peace region have combined to NORTHERN LEOPARD FROGS GET THEIR form a single newsletter that now includes projects from the Coastal region. Take a look inside for some amazing stories. OWN INSURANCE POLICY The Fish and Wildlife Compensation Program (FWCP) has teamed up with the Vancouver Aquarium to create a very unusual insurance policy. IN THIS ISSUE Over the last two years, with the help of the FWCP, 113 northern leopard frog tadpoles have been moved from the Release of marmot pups 2 Creston Valley Wildlife Management Area in southeast B.C. to the Vancouver Aquarium. Biologists hope that some of the 60 adults currently in captivity will successfully breed in the future. This is one of many projects the FWCP has taken part in on behalf of its program partners BC Hydro, the Province of B.C. and Fisheries and Oceans Canada who Message from the partners 3 work together to conserve and enhance fish and wildlife in British Columbia. “The goal is to maintain a back-up population should northern leopard frogs disappear from the wetlands of British Expanding the goat population 3 Columbia,” says Dr. -

CAA/ACA Bulletin
CAA/ACA Bulletin Volume 22, Issue 1, Spring 2002 A Message from the President. Un Message du Président. Gerry Oetelaar This is my last message as president of Voici mon dernier message comme président de the association and the content is any- lassociation et le contenu nest pas très agréable. thing but uplifting. In her final com- Lors de son discours final, il y a quelques années, munication as president a couple of Mima nous a indiqué que sa contribution principale years ago, Mima justifiably summa- comme présidente était de placer les affaires de rized her single most important con- lassociation en ordre. Je minquiète que ma contri- tribution as getting the CAAs house bution envers lassociation ces dernières années sera in order. I am afraid that my contri- vue comme linverse. Malgré mes intentions les plus bution to the association will be viewed as the sincères, jai vu les affaires sécrouler devant mes Association exact opposite. Despite the best of intentions, I yeux - ladhésion des membres est demeurée la News have watched the house collapse around me - même, lassemblée annuelle 2002 a du changer de membership has remained un- le lieu de réunion à la dernière seconde, Issue changed, the 2002 conference et récemment notre demande de changed venues at the last minute, “It is with bourse a été reniée. Chacuns de ces and, most recently, our grant appli- regret that I points a une implication financière cation was rejected. Each of these auprès de lassociation, mais jaimerais has serious financial repercussions must inform me concentrer sur notre demande de for the Canadian Archaeological As- you that the bourse. -

Carrier Sekani Tribal Council Aboriginal Interests & Use Study On
Carrier Sekani Tribal Council Aboriginal Interests & Use Study on the Enbridge Gateway Pipeline An Assessment of the Impacts of the Proposed Enbridge Gateway Pipeline on the Carrier Sekani First Nations May 2006 Carrier Sekani Tribal Council i Aboriginal Interests & Use Study on the Proposed Gateway Pipeline ACKNOWLEDGEMENTS The Carrier Sekani Tribal Council Aboriginal Interests & Use Study was carried out under the direction of, and by many members of the Carrier Sekani First Nations. This work was possible because of the many people who have over the years established the written records of the history, territories, and governance of the Carrier Sekani. Without this foundation, this study would have been difficult if not impossible. This study involved many community members in various capacities including: Community Coordinators/Liaisons Ryan Tibbetts, Burns Lake Band Bev Ketlo, Nadleh Whut’en First Nation Sara Sam, Nak’azdli First Nation Rosa McIntosh, Saik’uz First Nation Bev Bird & Ron Winser, Tl’azt’en Nation Michael Teegee & Terry Teegee, Takla Lake First Nation Viola Turner, Wet’suwet’en First Nation Elders, Trapline & Keyoh Holders Interviewed Dick A’huille, Nak’azdli First Nation Moise and Mary Antwoine, Saik’uz First Nation George George, Sr. Nadleh Whut’en First Nation Rita George, Wet’suwet’en First Nation Patrick Isaac, Wet’suwet’en First Nation Peter John, Burns Lake Band Alma Larson, Wet’suwet’en First Nation Betsy and Carl Leon, Nak’azdli First Nation Bernadette McQuarry, Nadleh Whut’en First Nation Aileen Prince, Nak’azdli First Nation Donald Prince, Nak’azdli First Nation Guy Prince, Nak’azdli First Nation Vince Prince, Nak’azdli First Nation Kenny Sam, Burns Lake Band Lillian Sam, Nak’azdli First Nation Ruth Tibbetts, Burns Lake Band Ryan Tibbetts, Burns Lake Band Joseph Tom, Wet’suwet’en First Nation Translation services provided by Lillian Morris, Wet’suwet’en First Nation. -

Geological Branch Assesssent Ztwppbt Table of Contents
I 1984 Assessment Report I Geological and Geochemical Surveys Claim: TREASURE MOUNTAIN Commodity: Copper, Gold Location: Kanaka Creek 10 Km NE of Haney 92G 8W 122' RbtW; ~f9~17- New Westminster M.D. Consul tant L. Sookochoff, P.Eng and Sookochoff Consultants Inc. Author: 31 1-409 Granville Street Vancouver, B.C. , V6C 1T2 Owner and MODULE RESOURCES INC. Operator: Vancouver , B.C. Work Dates: August 13, 1984 to August 23, 1984 Submittal Date: October 2, 1984. GEOLOGICAL BRANCH ASSESSSENT ZTWPPBT TABLE OF CONTENTS INTRODUCTION ---,-------------------------------------------- 1- / PROPERTY ................................................... lo/ LOCATION AND ACCESS ........................................ 2 *< PHYSIOGRAPHY ............................................... 2*/ WATER AND POWER ............................................ 2 *' HISTORY .................................................... 2 */ GEOLOGY .................................................... 4 */ GEOCHEMICAL SURVEY ......................................... 5 ./ RESULTS OF THE 1984 EXPLORATION PROGRAM .................... 6 J CONCLUSIONS ................................................ 8 r RECOMMENDATIONS ............................................ 8 / BIBLIOGRAPHY ............................................... 9 r CERTIFICATE ................................................ 10, STATEMENT OF COSTS ......................................... 11 / ILLUSTRATIONS FIGURE 1 GEOLOGY & CLAIM MAP FIGURE 2 INDEX & CLAIM MAP FIGURE 3 GEOLOGY MAP FIGURE 4 ARSENIC GEOCHEM MAP FIGURE -

Snow Survey and Water Supply Bulletin – January 1St, 2021
Snow Survey and Water Supply Bulletin – January 1st, 2021 The January 1st snow survey is now complete. Data from 58 manual snow courses and 86 automated snow weather stations around the province (collected by the Ministry of Environment Snow Survey Program, BC Hydro and partners), and climate data from Environment and Climate Change Canada and the provincial Climate Related Monitoring Program have been used to form the basis of the following report1. Weather October began with relatively warm and dry conditions, but a major cold spell dominated the province in mid-October. Temperatures primarily ranged from -1.5 to +1.0˚C compared to normal. The cold spell also produced early season low elevation snowfall for the Interior. Following the snowfall, heavy rain from an atmospheric river affected the Central Coast and spilled into the Cariboo, resulting in prolonged flood conditions. Overall, most of the Interior received above normal precipitation for the month, whereas coastal regions were closer to normal. In November, temperatures were steady at near normal to slightly above normal and primarily ranged from -0.5 to +1.5˚C through the province. The warmest temperatures relative to normal occurred in the Interior, while the coldest occurred in the Northwest. Precipitation was mostly below normal to near normal (35-105%) with the Northeast / Peace as the driest areas. A few locations, e.g. Prince Rupert and Williams Lake, were above 130% due to a strong storm event early in the month. Temperatures in December were relatively warm across the province, ranging from +1.0 to +5.0˚C above normal. -

BC Hydro Dam Safety Quarterly Report
Confidential - Discussion/Information Board briefing – DAM SAFETY QUARTERLY REPORT Executive Summary The purpose of this report is to update the Capital Projects Committee of the Board of Directors on key dam risk management activities during the period from April 1, 2019 to June 30, 2019, and to provide reasonable assurance that the safety of dams operated by BC Hydro continues to be managed to the established guidelines and criteria of the Dam Safety Program. The Dam Safety Program has been executed in a manner that is consistent with its stated objectives throughout the reporting period. The overall Dam Safety risk profile is shown in Figure 1. There have been no changes in assessed risk this quarter. Risk Profile of BC Hydro’s Dam Dam Safety Contribution to Enterprise Risk Dam Safety is assigned a high “risk priority” within BC Hydro’s Enterprise Risk report, as depicted below. This high rating is arrived at by recognizing that: (1) there can be extremely severe consequences from the failure of a dam; (2) a dam failure can progress quickly without leaving adequate time to take effective actions to reverse the failure; and (3) our ability to mitigate this risk is considered to be “moderate” given that upgrades to existing dams are typically expensive, time and resource intensive and frequently technically challenging. The nature of dam safety risk is that it can only be realistically managed by minimizing to the extent practicable the probability of occurrence through a well-constructed and well-executed Dam Safety Program. Speed Ability F19 Q4 Change from Risk Severity of to Risk Last Quarter Onset Mitigate Priority Likelihood Dam Safety For F20 Q1 the overall H L Fast M H Dam Safety risk is Risk of a dam safety incident stable. -

Wahleach Reservoir Fertilization Program
Wahleach Project Water Use Plan Wahleach Reservoir Fertilization Program Implementation Year 8 Reference: WAHWORKS #2 Wahleach Reservoir Nutrient Restoration Project, 2013-2014 Study Period: 2013-2014 Province of British Columbia, Ministry of Environment, Ecosystems Protection & Sustainability Branch December 2015 WAHLEACH RESERVOIR NUTRIENT RESTORATION PROJECT, 2013-2014 by A.S. Hebert1, S.L. Harris1, T. Weir2, M.B. Davies, and A. Schellenberg 1Ministry of Environment, Conservation Science Section, 315 - 2202 Main Mall, University of British Columbia, Vancouver, BC V6T 1Z4 2Ministry of Forests, Lands, and Natural Resource Operations, Fish, Wildlife and Habitat Management Branch, 4th Floor - 2975 Jutland Road, Victoria, BC V8T 5J9 Fisheries Project Report No. RD153 2015 Province of British Columbia Ministry of Environment Ecosystems Protection & Sustainability Branch Copyright Notice No part of the content of this document may be reproduced in any form or by any means, including storage, reproduction, execution, or transmission without the prior written permission of the Province of British Columbia. Limited Exemption to Non-reproduction Permission to copy and use this publication in part, or in its entirety, for non-profit purposes within British Columbia, is granted to BC Hydro; and Permission to distribute this publication, in its entirety, is granted to BC Hydro for non-profit purposes of posting the publication on a publicly accessible area of the BC Hydro website. Wahleach Reservoir Nutrient Restoration Project, 2013-2014 ii Data and information contained within this data report are considered preliminary and subject to change. Wahleach Reservoir Nutrient Restoration Project, 2013-2014 iii Acknowledgements This project was completed by the Ministry of Environment, Conservation Science Section under a Memorandum of Understanding with BC Hydro. -

Revised Draft Experiences with Inter Basin Water
REVISED DRAFT EXPERIENCES WITH INTER BASIN WATER TRANSFERS FOR IRRIGATION, DRAINAGE AND FLOOD MANAGEMENT ICID TASK FORCE ON INTER BASIN WATER TRANSFERS Edited by Jancy Vijayan and Bart Schultz August 2007 International Commission on Irrigation and Drainage (ICID) 48 Nyaya Marg, Chanakyapuri New Delhi 110 021 INDIA Tel: (91-11) 26116837; 26115679; 24679532; Fax: (91-11) 26115962 E-mail: [email protected] Website: http://www.icid.org 1 Foreword FOREWORD Inter Basin Water Transfers (IBWT) are in operation at a quite substantial scale, especially in several developed and emerging countries. In these countries and to a certain extent in some least developed countries there is a substantial interest to develop new IBWTs. IBWTs are being applied or developed not only for irrigated agriculture and hydropower, but also for municipal and industrial water supply, flood management, flow augmentation (increasing flow within a certain river reach or canal for a certain purpose), and in a few cases for navigation, mining, recreation, drainage, wildlife, pollution control, log transport, or estuary improvement. Debates on the pros and cons of such transfers are on going at National and International level. New ideas and concepts on the viabilities and constraints of IBWTs are being presented and deliberated in various fora. In light of this the Central Office of the International Commission on Irrigation and Drainage (ICID) has attempted a compilation covering the existing and proposed IBWT schemes all over the world, to the extent of data availability. The first version of the compilation was presented on the occasion of the 54th International Executive Council Meeting of ICID in Montpellier, France, 14 - 19 September 2003. -

Reduced Annualreport1972.Pdf
PROVINCE OF BRITISH COLUMBIA DEPARTMENT OF RECREATION AND CONSERVATION HON. ROBERT A. WILLIAMS, Minister LLOYD BROOKS, Deputy Minister REPORT OF THE Department of Recreation and Conservation containing the reports of the GENERAL ADMINISTRATION, FISH AND WILDLIFE BRANCH, PROVINCIAL PARKS BRANCH, BRITISH COLUMBIA PROVINCIAL MUSEUM, AND COMMERCIAL FISHERIES BRANCH Year Ended December 31 1972 Printed by K. M. MACDONALD, Printer to tbe Queen's Most Excellent Majesty in right of the Province of British Columbia. 1973 \ VICTORIA, B.C., February, 1973 To Colonel the Honourable JOHN R. NICHOLSON, P.C., O.B.E., Q.C., LLD., Lieutenant-Governor of the Province of British Columbia. MAY IT PLEASE YOUR HONOUR: Herewith I beg respectfully to submit the Annual Report of the Department of Recreation and Conservation for the year ended December 31, 1972. ROBERT A. WILLIAMS Minister of Recreation and Conservation 1_) VICTORIA, B.C., February, 1973 The Honourable Robert A. Williams, Minister of Recreation and Conservation. SIR: I have the honour to submit the Annual Report of the Department of Recreation and Conservation for the year ended December 31, 1972. LLOYD BROOKS Deputy Minister of Recreation and Conservation CONTENTS PAGE Introduction by the Deputy Minister of Recreation and Conservation_____________ 7 General Administration_________________________________________________ __ ___________ _____ 9 Fish and Wildlife Branch____________ ___________________ ________________________ _____________________ 13 Provincial Parks Branch________ ______________________________________________ -

Habitat-Based Methods to Estimate Spawner Capacity for Chinook
C S A S S C C S Canadian Science Advisory Secretariat Secrétariat canadien de consultation scientifique Research Document 2002/114 Document de recherche 2002/114 Not to be cited without Ne pas citer sans permission of the authors * autorisation des auteurs * Habitat-based methods to estimate Méthodes axées sur l’habitat pour estimer spawner capacity for chinook salmon in la capacité d’accueil de saumons the Fraser River watershed quinnats géniteurs dans le réseau fluvial du fleuve Fraser C. K. Parken1 , J. R. Irvine1, R. E. Bailey2, I. V. Williams3 1Fisheries and Oceans Canada Science Branch, Stock Assessment Division Pacific Biological Station Nanaimo, B.C. V9T 6N7 2Fisheries and Oceans Canada BC Interior, Resource Management 1278 Dalhousie Drive Kamloops, B.C. V2B 6G3 3I.V. Williams Consulting Ltd. 3565 Planta Rd. Nanaimo, B.C. V9T 1M1 * This series documents the scientific basis for the * La présente série documente les bases scientifiques evaluation of fisheries resources in Canada. As such, des évaluations des ressources halieutiques du Canada. it addresses the issues of the day in the time frames Elle traite des problèmes courants selon les échéanciers required and the documents it contains are not dictés. Les documents qu’elle contient ne doivent pas intended as definitive statements on the subjects être considérés comme des énoncés définitifs sur les addressed but rather as progress reports on ongoing sujets traités, mais plutôt comme des rapports d’étape investigations. sur les études en cours. Research documents are produced in the official Les documents de recherche sont publiés dans la language in which they are provided to the langue officielle utilisée dans le manuscrit envoyé au Secretariat. -
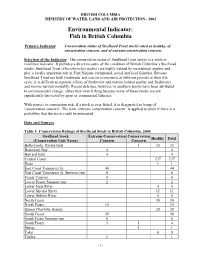
Fish 2002 Tec Doc Draft3
BRITISH COLUMBIA MINISTRY OF WATER, LAND AND AIR PROTECTION - 2002 Environmental Indicator: Fish in British Columbia Primary Indicator: Conservation status of Steelhead Trout stocks rated as healthy, of conservation concern, and of extreme conservation concern. Selection of the Indicator: The conservation status of Steelhead Trout stocks is a state or condition indicator. It provides a direct measure of the condition of British Columbia’s Steelhead stocks. Steelhead Trout (Oncorhynchus mykiss) are highly valued by recreational anglers and play a locally important role in First Nations ceremonial, social and food fisheries. Because Steelhead Trout use both freshwater and marine ecosystems at different periods in their life cycle, it is difficult to separate effects of freshwater and marine habitat quality and freshwater and marine harvest mortality. Recent delcines, however, in southern stocks have been attributed to environmental change, rather than over-fishing because many of these stocks are not significantly harvested by sport or commercial fisheries. With respect to conseration risk, if a stock is over fished, it is designated as being of ‘conservation concern’. The term ‘extreme conservation concern’ is applied to stock if there is a probablity that the stock could be extirpated. Data and Sources: Table 1. Conservation Ratings of Steelhead Stock in British Columbia, 2000 Steelhead Stock Extreme Conservation Conservation Healthy Total (Conservation Unit Name) Concern Concern Bella Coola–Rivers Inlet 1 32 33 Boundary Bay 4 4 Burrard