Poly(3,3-Dimethylselenetane)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent (19) 11 Patent Number: 4,755,589 Sandman (45) Date of Patent: Jul
United States Patent (19) 11 Patent Number: 4,755,589 Sandman (45) Date of Patent: Jul. 5, 1988 (54) AROMATIC SELENUM COMPOUND POLYMER OTHER PUBLICATIONS Balodia et al., J. Org. Chem. USSR (Engl. Transl.) 15, 75 Inventor: Daniel J. Sandman, Acton, Mass. 343 (1979). Gladysz et al., J. Org. Chem. 43, 1204 (1978). Assignee: GTE Laboratories Incorporated, Battistoni, P. et al., Gazz. Chim, Ital. III, 505 (1981). 73 Sandman, D. J. et al. (1982) J. Chen. Soc., Chem. Com Waltham, Mass. mun. pp. 1133-1134. Ohnishi, S. et al. (1982) Chemistry Letters, pp. (21) Appl. No.: 870,122 1841-1842. Endres, H. et al. (1982) Mol. Cryst. Liq. Cryst. 86 22 Filed: Jun. 3, 1986 111-122. K. Y. Jen et al., J. Polymer Sci., Polymer Lett. 21, 441 (1983). Related U.S. Application Data S. Tanaka, et al., Makromol. Chem., Rapid Commun. 4, 62 Division of Ser. No. 507,156, Jun. 23, 1983, Pat. No. 231-235 (1983). 4,597,914. T. Hasegawa et al., J. Polymer Sci., Polymer Lett. 22, 365 (1984). (51) Int. Cl* .............................................. CO8G 83/00 Primary Examiner-Harold D. Anderson 52) U.S. C. ................. ... 528/397; 528/388 Attorney, Agent, or Firm-Hamilton, Brook, Smith & 58 Field of Search ...................... 528/397,388 Reynolds 56) References Cited 57 ABSTRACT U.S. PATENT DOCUMENTS This invention constitutes a method for preparing mo lecular and polymeric aromatic selenide compounds 3,149,101 9/1964 Hubel et al. ........................ 260/239 such as bis-phenyl selenide and poly(p-phenylene sele 3,354,129 11/1967 Edmonds et al. ................... 528/265 3,790,536 2/1974 Vidaurri............................. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

1. Give the Correct Names for Each of the Compounds Listed Below. A
1. Give the correct names for each of the compounds listed below. a) NaCl sodium chloride n) ZrS2 zirconium sulfide b) FrBr francium bromide o) AgI silver iodide c) KF potassium fluoride p) BaSe barium selenide d) RaS radium sulfide q) MgO magnesium oxide e) LiI lithium iodide r) LaBr3 lanthanum bromide f) Li3N lithium nitride s) Sr3N2 strontium nitride g) AlBr3 aluminum bromide t) Cd3As2 cadmium arsenide h) CdCl2 cadmium chloride u) Rb2Se rubidium selenide i) K2O potassium oxide v) Rb3N rubidium nitride j) InF3 indium fluoride w) BaF2 barium fluoride k) ZnO zinc oxide x) ZrTe2 zirconium telluride l) Y2O3 yttrium oxide y) Cs3P cesium phosphide m) CaTe calcium telluride z) Y2O3 yttrium oxide 2. Write the correct chemical formula for each of the following compounds. a) potassium bromide KBr n) potassium nitride K3N b) zinc bromide ZnBr2 o) aluminum bromide AlBr3 c) lithium iodide LiI p) zinc phosphide Zn3P2 d) scandium chloride ScCl3 q) magnesium sulfide MgS e) magnesium chloride MgCl2 r) hafnium chloride HfCl4 f) magnesium oxide MgO s) barium sulfide BaS g) hydrogen sulfide H2S t) tantalum oxide Ta2O5 h) gallium iodide GaI3 u) zirconium nitride Zr3N4 i) sodium oxide Na2O v) potassium selenide K2Se j) magnesium selenide MgSe w) germanium fluoride GeF4 k) calcium fluoride CaF2 x) francium phosphide Fr3P l) aluminum oxide Al2O3 y) zinc arsenide Zn3As2 m) beryllium chloride BeCl2 z) scandium telluride Sc2Te3 L. h. s. – Chemistry – Nomenclature – Answers – Page 1 3. Give the correct names for each of the compounds listed below. a) CaSO4 calcium -

STATES PATENT of FICE 2,414,438 Electrodeposition of SELENIUM Mortimer C
Patented Jan. 21, 1947. 2,414,438 UNITED STATES PATENT of FICE 2,414,438 ELECTRoDEPoSITION OF SELENIUM Mortimer C. Bloom, Newton Highlands, Mass, as signor, by mesne assignments, to Federal Tele phone and Radio Corporation, New York, N.Y., a corporation of Delaware No Drawing. Application December 1, 1942, Serial No. 467,562 4. Claims. (C. 204-56) 2 This invention relates to the electro-deposition Another way of preparing the solution is to of Selenium and has for its object to provide a, dissolve powdered selenium in a solution of SO method of depositing metallic selenium evenly in dium hydroxide. This will form the desired so a pure form and without the use of corrosive SO dium selenide (Na2Se2). It will form, in addi lutionS. tion, sodium selenite (Na2Set4O3) which is use Another object is to attain an even and fine less for the purpose of anodic plating as the --4 grained selenium deposit on a base element which ions will pass to the cathode rather than the will be useful for a rectifier or photocell. anode. Consequently, it is preferred to use a so Selenium has heretofore been electro-deposited lution containing only the desired selenide and cathodically by the use of solutions producing O not the selenite; and the selenide free from the quadrivalent selenium cations (Se). Such a selenite can be had by the method of treating Condition is had in a selenious acid solution Con with the gas H2Se, taining an excess of acid as disclosed and claimed For the anode almost any conducting plate or in copending application Ser. -

Standard X-Ray Diffraction Powder Patterns NATIONAL BUREAU of STANDARDS
NBS MONOGRAPH 25—SECTION 1 9 CO Q U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Standard X-ray Diffraction Powder Patterns NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau's technical work is per- formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system of physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essentia! services leading to accurate and uniform physical and chemical measurement throughout the Nation's scientific community, industry, and commerce; conducts materials research leading to improved methods of measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes Standard Reference Materials; and provides calibration -

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

Molecular and Supramolecular Chemistry of Mono- and Di-Selenium
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Sunway Institutional Repository Molecular and supramolecular chemistry of mono- and di-selenium analogues of metal dithiocarbamates See Mun Lee,a Peter J. Heardb and Edward R. T. Tiekinka,* a Research Centre for Crystalline Materials, School of Science and Technology, Sunway University, No. 5 Jalan Universiti, 47500 Bandar Sunway, Selangor Darul Ehsan, Malaysia b Office of the Provost, Sunway University, No. 5 Jalan Universiti, 47500 Bandar Sunway, Selangor Darul Ehsan, Malaysia ⁎ Corresponding author. Email address: [email protected] (E.R.T. Tiekink) Received: ABSTRACT This bibliographic review summarises the coordination chemistry of mono- and di- - selenium analogues of metal dithiocarbamate ligands, [RRꞌNCS2] , as revealed by X-ray crystallography and spectroscopy (77Se NMR and infrared). The Se-ligands are usually chelating but, bridging modes, up to 4, are known. Reflecting the larger size, greater polarisability and presence of a polar-cap (-hole), selenium atoms are more likely to be involved in secondary-bonding (chalcogen-bonding) than sulphur when a competition exists. Isostructural relationships are established across the series in about one-third of the structures. Keywords: Selenothiocarbamate; Diselenocarbamate; Dithiocarbamate; Structures; Supramolecular chemistry; Secondary-bonding; 77Se NMR 2 Contents 1. Introduction 2. Selenothiocarbamates 2.1 Structures of oxidation products 2.2 Alkali metal salt 2.3 Transition metal complexes 2.4 Main group element compounds 3. Diselenocarbamates 3.1 Spectroscopic data 3.2 Transition metal complexes 3.2.1 Iron complex 3.2.2 Cobalt complex 3.2.3 Nickel group complexes 3.2.4 Copper complexes 3.3 Main group element compounds 3.3.1 Zinc structures 3.3.2 Cadmium structures 3.3.3 Indium structures 3.3.4 Lead(II) structure 3.3.5 Bismuth structure 3.3.6 Selenium structures 4 Relationship to dithiocarbamate structures Conclusions and outlook References 3 1. -
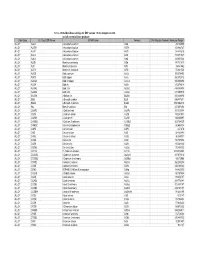
Database Full Listing
16-Nov-06 OLI Data Base Listings for ESP version 7.0.46, Analyzers 2.0.46 and all current alliance products Data Base OLI Tag (ESP) Name IUPAC Name Formula CAS Registry Number Molecular Weight ALLOY AL2U 2-Aluminum uranium Al2U 291.98999 ALLOY AL3TH 3-Aluminum thorium Al3Th 312.982727 ALLOY AL3TI 3-Aluminum titanium Al3Ti 128.824615 ALLOY AL3U 3-Aluminum uranium Al3U 318.971527 ALLOY AL4U 4-Aluminum uranium Al4U 345.953064 ALLOY ALSB Aluminum antimony AlSb 148.731537 ALLOY ALTI Aluminum titanium AlTi 74.861542 ALLOY ALTI3 Aluminum 3-titanium AlTi3 170.621536 ALLOY AUCD Gold cadmium AuCd 309.376495 ALLOY AUCU Gold copper AuCu 260.512512 ALLOY AUCU3 Gold 3-copper AuCu3 387.604492 ALLOY AUSN Gold tin AuSn 315.676514 ALLOY AUSN2 Gold 2-tin AuSn2 434.386505 ALLOY AUSN4 Gold 4-tin AuSn4 671.806519 ALLOY BA2SN 2-Barium tin Ba2Sn 393.369995 ALLOY BI2U 2-Bismuth uranium Bi2U 655.987671 ALLOY BI4U3 4-Bismuth 3-uranium Bi4U3 1550.002319 ALLOY BIU Bismuth uranium BiU 447.007294 ALLOY CA2PB 2-Calcium lead Ca2Pb 287.355988 ALLOY CA2SI 2-Calcium silicon Ca2Si 108.241501 ALLOY CA2SN 2-Calcium tin Ca2Sn 198.865997 ALLOY CA3SB2 3-Calcium 2-antimony Ca3Sb2 363.734009 ALLOY CAMG2 Calcium 2-magnesium CaMg2 88.688004 ALLOY CAPB Calcium lead CaPb 247.278 ALLOY CASI Calcium silicon CaSi 68.163498 ALLOY CASI2 Calcium 2-silicon CaSi2 96.249001 ALLOY CASN Calcium tin CaSn 158.787994 ALLOY CAZN Calcium zinc CaZn 105.468002 ALLOY CAZN2 Calcium 2-zinc CaZn2 170.858002 ALLOY CD11U 11-Cadmium uranium Cd11U 1474.536865 ALLOY CD3AS2 3-Cadmium 2-arsenic As2Cd3 487.073212 -

Department of Labor
Vol. 79 Friday, No. 197 October 10, 2014 Part II Department of Labor Occupational Safety and Health Administration 29 CFR Parts 1910, 1915, 1917, et al. Chemical Management and Permissible Exposure Limits (PELs); Proposed Rule VerDate Sep<11>2014 17:41 Oct 09, 2014 Jkt 235001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\10OCP2.SGM 10OCP2 mstockstill on DSK4VPTVN1PROD with PROPOSALS2 61384 Federal Register / Vol. 79, No. 197 / Friday, October 10, 2014 / Proposed Rules DEPARTMENT OF LABOR faxed to the OSHA Docket Office at Docket: To read or download (202) 693–1648. submissions or other material in the Occupational Safety and Health Mail, hand delivery, express mail, or docket go to: www.regulations.gov or the Administration messenger or courier service: Copies OSHA Docket Office at the address must be submitted in triplicate (3) to the above. All documents in the docket are 29 CFR Parts 1910, 1915, 1917, 1918, OSHA Docket Office, Docket No. listed in the index; however, some and 1926 OSHA–2012–0023, U.S. Department of information (e.g. copyrighted materials) Labor, Room N–2625, 200 Constitution is not publicly available to read or [Docket No. OSHA 2012–0023] Avenue NW., Washington, DC 20210. download through the Web site. All submissions, including copyrighted RIN 1218–AC74 Deliveries (hand, express mail, messenger, and courier service) are material, are available for inspection Chemical Management and accepted during the Department of and copying at the OSHA Docket Office. Permissible Exposure Limits (PELs) Labor and Docket Office’s normal FOR FURTHER INFORMATION CONTACT: business hours, 8:15 a.m. -

Standard X-Ray Diffraction Powder Patterns
7 NATL INST OF STANDARDS & TECH R.I.C. Nes AlllOD Ififib^fi PUBLICATIONS 100988698 ST5°5rv'25-17;1980 C.1 NBS-PUB-C 19 cr»T OF NBS MONOGRAPH 25-SECTION 1 V) J U.S. DEPARTMENT OF COMMERCE / National Bureau of Standards Standard X-ray Diffraction Powder Patterns . NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act ot Congress on March 3, 1901 The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau's technical work is per- formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system of physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essential services leading to accurate and uniform physical and chemical measurement throughout the Nation's scientific community, industry, and commerce; conducts materials research leading to improved methods ol measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes Standard Reference Materials; and provides calibration services. The Laboratory consists of the following centers: Absolute Physical Quantities- — Radiation Research — Thermodynamics and Molecular Science — Analytical Chemistry — Materials Science. -

Ionic Bonding -Binary Making an Ionic Compound Once the Number Of
Ionic Bonding -Binary Making an Ionic Compound Once the number of electrons that will be gained or lost is known, the cation and anion are put together to make a compound. Because ionic compounds are neutral overall, the number of the positives from the cation must balance with the number of negatives from the anion. If lithium and fluorine bond, Li+ and F- would make LiF, because the positive 1 charge balances a negative 1 charge. But if lithium (Li+) and oxygen (O-2) bond, the compound would end up being LiO-. Obviously more positive lithiums are needed to balance out the larger negative of oxygen. It would take 2 lithiums for every 1 oxygen. To show two lithiums are needed, a subscript of “2” is written after the lithium, Li2O (do not write subscripts of 1). If an ionic compound is made from Aluminum (Al+3) and Sulfur (S-2), the amounts of each element needed would be: Al+3 S-2 Al+3 S-2 S-2 Totals: +6 and -6 So the resulting compound would be Al2S3. If you look at the subscript on the aluminum, you’ll see it matches the negative number from the sulfur, and the subscript on the sulfur matches the positive number from the aluminum. This trend is used as a shortcut, called the criss-cross method. The number from the metal becomes the subscript of the nonmetal, and the number of the nonmetal becomes the subscript of the metal. The only time this method has a problem is with numbers that are the same. -

Modern Chemistry Name Chapter 7 Chemical Formulas and Chemical Compounds
Modern Chemistry Name Chapter 7 Chemical Formulas and Chemical Compounds Type I Binary Ionic Compounds Name the following compounds. Formula of compound Name of compound NaCl KBr Li2S AlCl3 RbF Ca2N3 Na2S LiCl SrO CsF MgI2 BeO KF Al2O3 NaF Write the formulas for the following compounds. Name of Compound Formula of compound Sodium oxide Lithium fluoride Magnesium chloride Calcium sulfide Potassium selenide Rubidium bromide Potassium iodide Strontium fluoride Cesium sulfide Barium bromide 4 Modern Chemistry Name Chapter 7 Chemical Formulas and Chemical Compounds Calcium phosphide Lithium nitride Barium sulfide Strontium iodide Rubidium chloride Type II Binary Ionic Compounds Write the formulas for the following compounds. Name of compound Formula of compound Iron III oxide Lead IV oxide Tin II sulfide Manganese II iodide Chromium III chloride Molybdenum IV bromide Nickel II chloride Copper I oxide Tungsten III sulfide Titanium III phosphide Lead IV fluoride Iridium II oxide Write the names of the following compounds (be sure to use Roman Numerals). Formula of compound Name of compound PbO CuF2 Ni2O3 Co3N2 CrS3 SnI4 PtO 5 Modern Chemistry Name Chapter 7 Chemical Formulas and Chemical Compounds CuO Nb2O5 Fe2O3 PdCl2 VP Compounds Containing Polyatomic Ions Name the following compounds containing polyatomic ions. Formula of compound Name of compound NaClO2 K2SO3 (NH4)2CrO4 Al2(SO4)3 Ca(NO2)2 Fe(OH)3 KMnO4 PbSO4 Co(NO2)2 Mn(NO3)2 NaClO Li2S2O3 Write the formulas for the following compounds. Name of compound Formula of compound Copper II chromate Cobalt II acetate Lead II azide Copper I carbonate Cobalt II phosphate Iron III bromate 6 Modern Chemistry Name Chapter 7 Chemical Formulas and Chemical Compounds Ammonium oxalate Potassium periodate Barium arsenate Zinc tartrate Silver chromate Aluminum citrate Type III Compounds (Molecules) Name the following molecules.