Standard X-Ray Diffraction Powder Patterns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aldrich FT-IR Collection Edition I Library
Aldrich FT-IR Collection Edition I Library Library Listing – 10,505 spectra This library is the original FT-IR spectral collection from Aldrich. It includes a wide variety of pure chemical compounds found in the Aldrich Handbook of Fine Chemicals. The Aldrich Collection of FT-IR Spectra Edition I library contains spectra of 10,505 pure compounds and is a subset of the Aldrich Collection of FT-IR Spectra Edition II library. All spectra were acquired by Sigma-Aldrich Co. and were processed by Thermo Fisher Scientific. Eight smaller Aldrich Material Specific Sub-Libraries are also available. Aldrich FT-IR Collection Edition I Index Compound Name Index Compound Name 3515 ((1R)-(ENDO,ANTI))-(+)-3- 928 (+)-LIMONENE OXIDE, 97%, BROMOCAMPHOR-8- SULFONIC MIXTURE OF CIS AND TRANS ACID, AMMONIUM SALT 209 (+)-LONGIFOLENE, 98+% 1708 ((1R)-ENDO)-(+)-3- 2283 (+)-MURAMIC ACID HYDRATE, BROMOCAMPHOR, 98% 98% 3516 ((1S)-(ENDO,ANTI))-(-)-3- 2966 (+)-N,N'- BROMOCAMPHOR-8- SULFONIC DIALLYLTARTARDIAMIDE, 99+% ACID, AMMONIUM SALT 2976 (+)-N-ACETYLMURAMIC ACID, 644 ((1S)-ENDO)-(-)-BORNEOL, 99% 97% 9587 (+)-11ALPHA-HYDROXY-17ALPHA- 965 (+)-NOE-LACTOL DIMER, 99+% METHYLTESTOSTERONE 5127 (+)-P-BROMOTETRAMISOLE 9590 (+)-11ALPHA- OXALATE, 99% HYDROXYPROGESTERONE, 95% 661 (+)-P-MENTH-1-EN-9-OL, 97%, 9588 (+)-17-METHYLTESTOSTERONE, MIXTURE OF ISOMERS 99% 730 (+)-PERSEITOL 8681 (+)-2'-DEOXYURIDINE, 99+% 7913 (+)-PILOCARPINE 7591 (+)-2,3-O-ISOPROPYLIDENE-2,3- HYDROCHLORIDE, 99% DIHYDROXY- 1,4- 5844 (+)-RUTIN HYDRATE, 95% BIS(DIPHENYLPHOSPHINO)BUT 9571 (+)-STIGMASTANOL -

Bismuth Antimony Telluride
ci al S ence Mahajan et al., J Material Sci Eng 2018, 7:4 ri s te & a E M n DOI: 10.4172/2169-0022.1000479 f g o i n l e a e n r r i n u g o Journal of Material Sciences & Engineering J ISSN: 2169-0022 Research Article Article OpenOpen Access Access Study and Characterization of Thermoelectric Material (TE) Bismuth Antimony Telluride Aniruddha Mahajan1*, Manik Deosarkar1 and Rajendra Panmand2 1Chemical Engineering Department, Vishwakarma Institute of Technology, Pune, India 2Centre for Materials Electronics and Technology (C-MET), Dr. Homi Bhabha Road, Pune, India Abstract Thermoelectric materials are used to convert the heat to electricity with no moving parts, in the present work an attempt has been made to prepare it for power generation function. Bismuth antimony telluride nanopowders were prepared by using mechanochemical method. Three different materials; Bismuth Telluride, (Bi0.75Sb0.25)2Te 3 and (Bi0.5Sb0.5)2Te 3 were synthesized. XRD and TEM analysis was carried out to confirm the results. The particle size of the material was determined by using FESEM analysis. The two alloys of Bismuth Telluride such prepared were converted in the pellet form using vacuum hydraulic pressure and their Seebeck coefficients were determined to test the material suitability for its use as a thermoelectric device. Their power factor measurement and Hall effect measurements were carried out at room temperature. Keywords: Bismuth telluride; Mechanochemical method; energy in one form into another. Use of TE solid materials Applications Nanoparticals; Seebeck coefficients in heat pump and refrigeration is well known [14] and it is now expanded such as cooled seats in luxury automobiles [15]. -

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

Delayed Ettringite Formation
Ettringite Formation and the Performance of Concrete In the mid-1990’s, several cases of premature deterioration of concrete pavements and precast members gained notoriety because of uncertainty over the cause of their distress. Because of the unexplained and complex nature of several of these cases, considerable debate and controversy have arisen in the research and consulting community. To a great extent, this has led to a misperception that the problems are more prevalent than actual case studies would indicate. However, irrespective of the fact that cases of premature deterioration are limited, it is essential to address those that have occurred and provide practical, technically sound solutions so that users can confidently specify concrete in their structures. Central to the debate has been the effect of a compound known as ettringite. The objectives of this paper are: Fig. 1. Portland cements are manufactured by a process that combines sources of lime (such as limestone), silica and • to define ettringite and its form and presence in concrete, alumina (such as clay), and iron oxide (such as iron ore). Appropriately proportioned mixtures of these raw materials • to respond to questions about the observed problems and the are finely ground and then heated in a rotary kiln at high various deterioration mechanisms that have been proposed, and temperatures, about 1450 °C (2640 °F), to form cement compounds. The product of this process is called clinker • to provide some recommendations on designing for durable (nodules at right in above photo). After cooling, the clinker is concrete. interground with about 5% of one or more of the forms of Because many of the questions raised relate to cement character- calcium sulfate (gypsum shown at left in photo) to form portland cement. -

Breakthrough Chemistry Simulations for Lithium Processing Contents
think simulation | getting the chemistry right Breakthrough chemistry simulations for lithium processing Using simulation to maximize your investment return in lithium extraction and processing Contents Introduction ............................................................................................................................................ 2 Process simulation deficiencies ................................................................................................................ 2 OLI Systems electrolyte thermodynamics .................................................................................................2 OLI Systems lithium initiative .................................................................................................................... 2 Lithium phase 1 and potash chemistry is complete ................................................................................... 2 Fundamental sulfate – chloride systems .............................................................................................. 3 Fundamental hydroxide and carbonate systems .................................................................................. 3 Lithium in acid environments for processing and recycling ................................................................... 3 Lithium borate systems for Li production ............................................................................................. 4 Systems related to Li hydrometallurgical processing, purifications and recycling .................................. -

Bismuth Chloride Solution SDS US
SAFETY DATA SHEET Issue Date 10-Nov-2015 Revision Date 11-Nov-2015 Version 1 1. IDENTIFICATION Product identifier Product Name Bismuth Trichloride Solution Other means of identification Product Code 0650 UN/ID no. UN1760 Synonyms Bismuth chloride; Trichlorobismuth, Trichlorobismuthine Recommended use of the chemical and restrictions on use Recommended Use Laboratory chemicals. Uses advised against No information available Details of the supplier of the safety data sheet Manufacturer Address Harrell Industries, Inc. 2495 Commerce Drive Rock Hill, SC 29730 www.harrellindustries.com Emergency telephone number Company Phone Number 803-327-6335 Fax Number 803-327-7808 24 Hour Emergency Phone Number (800) 633-8253 PERS Emergency Telephone (800) 633-8253 (PERS) 2. HAZARDS IDENTIFICATION Classification OSHA Regulatory Status This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute toxicity - Inhalation (Gases) Category 4 Acute toxicity - Inhalation (Dusts/Mists) Category 4 Skin corrosion/irritation Category 1 Sub-category A Serious eye damage/eye irritation Category 1 Specific target organ toxicity (single exposure) Category 3 Label elements Emergency Overview Warning Hazard statements Corrosive to metals. Causes severe skin burns and eye damage May cause respiratory irritation _____________________________________________________________________________________________ Page 1 / 8 0650 - Bismuth Trichloride Solution Revision Date 11-Nov-2015 _____________________________________________________________________________________________ Appearance Clear, colorless to yellow Physical state liquid Odor Faint hydrochloric acid odor. liquid Precautionary Statements - Prevention Wash skin thoroughly after handling Wear eye protection/ face protection Wear protective gloves Precautionary Statements - Response Immediately call a POISON CENTER or doctor IF ON SKIN: Wash with plenty of soap and water IF IN EYES: Rinse cautiously with water for several minutes. -

Crown Chemical Resistance Chart
Crown Polymers, Corp. 11111 Kiley Drive Huntley, IL. 60142 USA www.crownpolymers.com 847-659-0300 phone 847-659-0310 facisimile 888-732-1270 toll free Chemical Resistance Chart Crown Polymers Floor and Secondary Containment Systems Products: CrownShield covers the following five (4) formulas: CrownShield 50, Product No. 320 CrownCote, Product No. 401 CrownShield 40-2, Product No. 323 CrownShield 28, Product No. 322 CrownPro AcidShield, Product No. 350 CrownCote AcidShield, Product No. 430 CrownPro SolventShield, Product No. 351 CrownCote SolventShield, Product No. 440 This chart shows chemical resistance of Crown Polymers foundational floor and secondary containment product line that would be exposed to chemical spill or immersion conditions. The chart was designed to provide general product information. For specific applications, contact your local Crown Polymers Floor and Secondary Containment Representative or call direct to the factory. ; Resistant to chemical immersion up to 7 days followed by wash down with water 6 Spillage environments that will be cleaned up within 72 hours after initial exposure. 9 Not Recommended Chemical CrownShield SolventShield AcidShield Chemical CrownShield SolventShield AcidShield 1, 4-Dichloro-2-butene 9 6 6 Aluminum Bromate ; ; ; 1, 4-Dioxane 9 6 6 Aluminum Bromide ; ; ; 1-1-1 Trichloroethane 9 ; ; Aluminum Chloride ; ; ; 2, 4-Pentanedione 6 ; 6 Aluminum Fluoride (25%) ; ; ; 3, 4-Dichloro-1-butene 6 6 6 Aluminum Hydroxide ; ; ; 4-Picoline (0-50%) 9 6 6 Aluminum Iodine ; ; ; Acetic Acid (0-15%) 9 6 6 -

Safety Data Sheet
Safety data sheet Page: 1/13 BASF Safety data sheet according to UN GHS 4th rev. Date / Revised: 23.10.2017 Version: 2.0 Product: Pearl-Glo® SF PG1099 (ID no. 30322522/SDS_COS_00/EN) Date of print 24.10.2017 1. Identification Product identifier Pearl-Glo® SF PG1099 Chemical name: Pearl-Glo SF CAS Number: 7787-59-9 Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses: cosmetic ingredient Details of the supplier of the safety data sheet Company: BASF SE 67056 Ludwigshafen GERMANY Telephone: +49 621 60-48799 E-mail address: [email protected] Emergency telephone number International emergency number: Telephone: +49 180 2273-112 2. Hazards Identification Classification of the substance or mixture According to UN GHS criteria No need for classification according to GHS criteria for this product. Page: 2/13 BASF Safety data sheet according to UN GHS 4th rev. Date / Revised: 23.10.2017 Version: 2.0 Product: Pearl-Glo® SF PG1099 (ID no. 30322522/SDS_COS_00/EN) Date of print 24.10.2017 Label elements Globally Harmonized System (GHS) The product does not require a hazard warning label in accordance with GHS criteria. Other hazards According to UN GHS criteria No specific dangers known, if the regulations/notes for storage and handling are considered. 3. Composition/Information on Ingredients Substances Chemical nature INCI Name: BISMUTH OXYCHLORIDE Contains: Bismuth chloride oxide CAS Number: 7787-59-9 EC-Number: 232-122-7 Hazardous ingredients (GHS) According to UN GHS criteria No particular hazards known. Mixtures Not applicable 4. First-Aid Measures Description of first aid measures Remove contaminated clothing. -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -
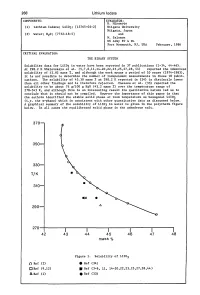
Evaluation of Aqueous Systems
268 Lithium Iodate COMPONENTS: EVALUATOR: H. Miyamoto (1) Lithium Iodate; LiI03; [13765-03-2] Niigata University Niigata, Japan (2) Water; H20; [7732-18-5] and M. Salomon US Army ET & DL Fort Monmouth, NJ, USA February, 1986 CRITICAL EVALUATION: THE BINARY SYSTEM Solubility data for LiI03 in water have been reported in 37 publications (1-34, 44-46). At 298.2 K Shklovskaya et al. (5,7,8,11,14-20,22,23,25,27,28,44) reported the identical solubility of 43.82 mass %, and although the work spans a period of 10 years (1974-1983), it is not possible to determine the number of independent measurements in these 18 publi cations. The solubility of 43.30 mass % at 298.2 K reported in (24) is distinctly lower than all other findings and is therefore rejected. Unezawa et al. (33) reported the solubility to be about 76 g/lOO g H20 (43.2 mass %) over the temperature range of 278-343 K, and although this is an interesting result its qualitative nature led us to conclude that it should not be compiled. However the importance of this paper is that the authors identified the stable solid phase at room temperature as hexagonal LiI03 (i.e. the a-phase) which is consistent with other quantitative data as discussed below. A graphical summary of the solubility of LiI03 in water is given in the polytherm figure below. In all cases the equilibrated solid phase is the anhydrous salt. 370 350 330 T/K 310 290 270-I------.r-----.-----..----......----------. 42 43 44 45 46 47 48 mass % Figure 1. -

Zinnwald Lithium Project
Zinnwald Lithium Project Report on the Mineral Resource Prepared for Deutsche Lithium GmbH Am St. Niclas Schacht 13 09599 Freiberg Germany Effective date: 2018-09-30 Issue date: 2018-09-30 Zinnwald Lithium Project Report on the Mineral Resource Date and signature page According to NI 43-101 requirements the „Qualified Persons“ for this report are EurGeol. Dr. Wolf-Dietrich Bock and EurGeol. Kersten Kühn. The effective date of this report is 30 September 2018. ……………………………….. Signed on 30 September 2018 EurGeol. Dr. Wolf-Dietrich Bock Consulting Geologist ……………………………….. Signed on 30 September 2018 EurGeol. Kersten Kühn Mining Geologist Date: Page: 2018-09-30 2/219 Zinnwald Lithium Project Report on the Mineral Resource TABLE OF CONTENTS Page Date and signature page .............................................................................................................. 2 1 Summary .......................................................................................................................... 14 1.1 Property Description and Ownership ........................................................................ 14 1.2 Geology and mineralization ...................................................................................... 14 1.3 Exploration status .................................................................................................... 15 1.4 Resource estimates ................................................................................................. 16 1.5 Conclusions and Recommendations ....................................................................... -

Nitrosamines EMEA-H-A5(3)-1490
25 June 2020 EMA/369136/2020 Committee for Medicinal Products for Human Use (CHMP) Assessment report Procedure under Article 5(3) of Regulation EC (No) 726/2004 Nitrosamine impurities in human medicinal products Procedure number: EMEA/H/A-5(3)/1490 Note: Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ...................................................................................... 2 1. Information on the procedure ............................................................... 7 2. Scientific discussion .............................................................................. 7 2.1. Introduction......................................................................................................... 7 2.2. Quality and safety aspects ..................................................................................... 7 2.2.1. Root causes for presence of N-nitrosamines in medicinal products and measures to mitigate them............................................................................................................. 8 2.2.2. Presence and formation of N-nitrosamines