Inorganic Chromium(Vi) Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Chromate 10 Percent Section 1
Conforms to US OSHA Hazard Communication 29CFR1910.1200 SAFETY DATA SHEET Sodium Chromate 10 percent Section 1. Identification 1.1 Product identifier Product name : Sodium Chromate 10 percent Part no. : AR176, AR376 Validation date : 6/24/2020 1.2 Relevant identified uses of the substance or mixture and uses advised against Material uses : Laboratory use Container type: Dispenser Pack AR176 // Sodium Chromate 10 percent // Artisan Grocott's Methenamine Silver Stain Kit // 65 mL and 115 mL AR376 // Sodium Chromate 10 percent // Artisan Grocott's Methenamine Silver Eosin Stain Kit // 65 mL and 115 mL Reference number: SDS056 1.3 Details of the supplier of the safety data sheet Supplier/Manufacturer : Agilent Technologies, Inc. 5301 Stevens Creek Blvd Santa Clara, CA 95051, USA Tel: +1 800 227 9770 Agilent Technologies Singapore (International) Pte Ltd. No. 1 Yishun Avenue 7 Singapore, 768923 Tel. (65) 6276 2622 Agilent Technologies Denmark ApS Produktionsvej 42 2600 Glostrup, Denmark Tel. +45 44 85 95 00 www.Agilent.com e-mail address of person : [email protected] responsible for this SDS 1.4 Emergency telephone number In case of emergency : CHEMTREC®: 1-800-424-9300 Section 2. Hazards identification 2.1 Classification of the substance or mixture OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the substance or mixture Date of issue : 06/24/2020 1/15 Sodium Chromate 10 percent Section 2. Hazards identification H302 ACUTE TOXICITY (oral) - Category 4 H311 -

Chemical Resistance Chart
CHEMICAL RESISTANCE CHART CHEMICAL RESISTANCE DATA These recommendations are based upon information from material suppliers and careful examination of available published information and are believed to be accurate. However, since the resistance of metals, plastics and elastomers can be affected by concentration, temperature, presence of other chemicals and other factors. This information should be considered as a general guide rather than an unqualified guarantee. Ultimately, the customer must determine the suitability of the pump used in various solutions. All recommendations assume ambient temperatures unless otherwise noted. RATINGS — CHEMICAL EFFECT FOOTNOTES A — No effect—Excellent 1. P.V.C. — Satisfactory to 72 °F B — Minor effect—Good 2. Polypropylene — Satisfactory to 72 °F C — Moderate effect—Fair 3. Polypropylene — Satisfactory to 120 °F D — Severe effect—Not recommended 4. Nitrile — Satisfactory for “O” Rings 5. Polyacetal — Satisfactory to 72 °F 6. Ceramag — Satisfactory to 72 °F The ratings for these materials are based upon the chemical resistance only. Added consideration must be given to pump selections when the chemical is abrasive, viscous in nature, or has a Specific Gravity greater than 1.1. NOTE: The materials shown below in BOLDFACE TYPE are used in the construction of Little Giant chemical pumps. ” 302 Stainless Steel 304 Stainless Steel 316 Stainless Steel 440 Stainless Steel Aluminum TITANIUM C276 NICKEL ALLOY Cast Bronze Brass Cast Iron Carbon Steel 1) PVC (Type (E-3606) Tygon PTFE Polyacetal Nylon ABS Thermoplastic -

HPLC for Food Analysis
HPLC for Food Analysis A Primer © Copyright Agilent Technologies Company, 1996-2001. All rights reserved. Reproduction, adaption, or translation without prior written permission is prohibited, except as allowed under the copyright laws. Printed in Germany www.agilent.com/chem September 01, 2001 Publication Number 5988-3294EN HPLC for Food Analysis A Primer The fundamentals of an alternative approach to solving tomorrow’s measurement challenges Angelika Gratzfeld-Hüsgen and Rainer Schuster Acknowledgements We would like to thank Christine Miller and John Jaskowiak for their contributions to this primer. Mrs. Miller is an application chemist with Agilent Technologies and is responsible for the material contained in chapter 5. Mr. Jaskowiak, who wrote chapter 7, is a product manager for liquid chromatography products at Agilent Technologies. © Copyright Agilent Technologies Company 1996-2001. All rights reserved. Reproduction, adaption, or translation without prior written permission is prohibited, except as allowed under the copyright laws. Printed in Germany, September 1, 2001. Publication Number 5988-3294EN Preface Modern agriculture and food processing often involve the use of chemicals. Some of these chemicals and their func- tions are listed below: • Fertilizers: increase production of agricultural plants • Pesticides: protect crops against weeds and pests • Antibiotics: prevent bacteria growth in animals during breeding • Hormones: accelerate animal growth • Colorants: increase acceptability and appeal of food • Preservatives and antioxidants: extend product life • Natural and artificial sweeteners and flavors: improve the taste of food • Natural and synthetic vitamins: increase the nutritive value of food • Carbohydrates: act as food binders Such chemicals improve productivity and thus increase competitiveness and profit margins. However, if the amounts consumed exceed certain limits, some of these chemicals may prove harmful to humans. -

Laboratory Study of Polychlorinated Biphenyl (PCB) Contamination and Mitigation in Buildings Part 4. Evaluation of the Activate
EPA/600/R-11/156C November 2012 Laboratory Study of Polychlorinated Biphenyl (PCB) Contamination and Mitigation in Buildings Part 4. Evaluation of the Activated Metal Treatment System (AMTS) for On-site Destruction of PCBs Xiaoyu Liu and Zhishi Guo U.S. Environmental Protection Agency Office of Research and Development National Risk Management Research Laboratory Air Pollution Prevention and Control Division Research Triangle Park, NC 27711 and Corey A. Mocka, R. Andy Stinson, Nancy F. Roache, and Joshua A. Nardin ARCADIS, US Inc. 4915 Prospectus Dr., Suite F Durham, NC 27709 NOTICE This document has been reviewed internally and externally in accordance with the U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. Executive Summary E.1 Background Polychlorinated biphenyls (PCBs) were once used as a plasticizer in certain building materials such as caulking, sealants, and paints from the 1950s through the late 1970s. Because PCBs have a variety of adverse health effects in animals and human, federal regulations have specific requirements for use and disposal of PCB-containing materials (U.S. EPA, 2005; 2009). Briefly, building materials that contain 50 ppm or more PCBs are not authorized for use and must be disposed of as PCB bulk product waste according the Code of Federal Regulations 40 CFR §761.3 and §761.62. If PCBs have contaminated either the surrounding building materials or adjacent soil, these materials are considered PCB remediation waste, which is subject to the cleanup and disposal requirements according 40 CFR §761.61. -

Protective Effect of Melatonin Against Chromium-Induced Hepatotoxic and Genotoxic Effect in Albino Rats
Global Veterinaria 16 (4): 323-329, 2016 ISSN 1992-6197 © IDOSI Publications, 2016 DOI: 10.5829/idosi.gv.2016.16.04.10332 Protective Effect of Melatonin Against Chromium-Induced Hepatotoxic and Genotoxic Effect in Albino Rats Emad A. Hashish and Shimaa A. Elgaml Department of Clinical Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Sharkyia, Egypt Abstract: Chromium is a widespread environmental waste. It is an industrial contaminant with teratogenic, mutagenic and carcinogenic effects on animals and human. This study was carried out to evaluate the potential protective effect of melatonin on the hepatotoxicity and genotoxicity generated by potassium dichromate (K2 Cr 27 O ) in albino rats. Rats were divided into four groups; control, melatonin (10 mg/kg b.wt.), melatonin pretreated with single S/C injection of K2 Cr 27 O (15 mg/kg b.wt.) and potassium dichromate treated group. Rats were sacrificed 24 h after K2 Cr 27 O treatment. K2 Cr 27 O treated rats showed significant (P<0.05) increase in the hepatic marker enzymes activity (aspartate aminotransferase-AST and alanine aminotransferase-ALT) and serum bilirubin (total and direct) was detected. Significant (P<0.05) decrease in the serum total protein and albumin was observed after K2 Cr 27 O treatment. Meanwhile, serum globulin, A/G ratio and indirect bilirubin showed no significant change. Hepatic DNA damage was observed using comet assay. The histological alterations confirmed the previous results. Melatonin pretreated rats showed an amelioration of the adverse effects of K2 Cr 27 O toxicity. An improvement in the serum hepatic enzymes (AST and ALT), proteinogram (total protein and albumin) and bilirubin (total and direct) was observed. -

Chemical Compatibility Guide
Chemical Compatibility Guide Guide Applicable to the Following: PIG Portable Spill Containment Pool Guide Information This report is offered as a guide and was developed from information which, to the best of New Pig’s knowledge, was reliable and accurate. Due to variables and conditions of application beyond New Pig’s control, none of the data shown in this guide is to be construed as a guarantee, expressed or implied. New Pig assumes no responsibility, obligation, or liability in conjunction with the use or misuse of the information. PIG Spill Containment Pools are constructed from PVC-coated polyester fabric. The chemical resistance guide that follows shows the chemical resistance for the PVC layer only. This guide has been compiled to provide the user with general chemical resistance information. It does not reflect actual product testing. Ratings / Key or Ratings – Chemical Effect 1. Satisfactory to 72°F (22°C) 2. Satisfactory to 120°F (48°C) A = Excellent D = Severe Effect, not recommended for ANY use. B = Good — Minor Effect, slight corrosion or discoloration. N/A = Information not available. C = Fair — Moderate Effect, not recommended for continuous use. Softening, loss of strength, swelling may occur. Due to variables and conditions beyond our control, New Pig cannot guarantee that this product(s) will work to your satisfaction. To ensure effectiveness and your safety, we recommend that you conduct compatibility and absorption testing of your chemicals with this product prior to purchase. For additional questions or information, -

Chromium Stress in Plants: Toxicity, Tolerance and Phytoremediation
sustainability Review Chromium Stress in Plants: Toxicity, Tolerance and Phytoremediation Dipali Srivastava 1,†, Madhu Tiwari 1,†, Prasanna Dutta 1,2, Puja Singh 1,2, Khushboo Chawda 1,2, Monica Kumari 1,2 and Debasis Chakrabarty 1,2,* 1 Biotechnology and Molecular Biology Division, CSIR-National Botanical Research Institute, Lucknow 226001, India; [email protected] (D.S.); [email protected] (M.T.); [email protected] (P.D.); [email protected] (P.S.); [email protected] (K.C.); [email protected] (M.K.) 2 Academy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Extensive industrial activities resulted in an increase in chromium (Cr) contamination in the environment. The toxicity of Cr severely affects plant growth and development. Cr is also recognized as a human carcinogen that enters the human body via inhalation or by consuming Cr-contaminated food products. Taking consideration of Cr enrichment in the environment and its toxic effects, US Environmental Protection Agency and Agency for Toxic Substances and Disease Registry listed Cr as a priority pollutant. In nature, Cr exists in various valence states, including Cr(III) and Cr(VI). Cr(VI) is the most toxic and persistent form in soil. Plants uptake Cr through various transporters such as phosphate and sulfate transporters. Cr exerts its effect by generating reactive oxygen species (ROS) and hampering various metabolic and physiological pathways. Studies Citation: Srivastava, D.; Tiwari, M.; on genetic and transcriptional regulation of plants have shown the various detoxification genes get Dutta, P.; Singh, P.; Chawda, K.; up-regulated and confer tolerance in plants under Cr stress. -
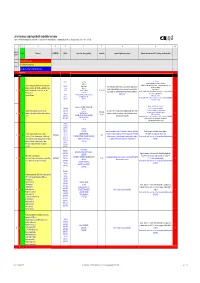
List of Substances Subject to Prohibited / Undesirable / Declaration
List of substances subject to prohibited / undesirable / declaration Appendix 1 to the Guidelines to Design and Purchasing Prohibition of Harmful Substances : ebmpapstMulfingen: 90-001 ebmpapstSt.Georgen: WN 3-12.2 ebmpapstLandshut: 60118.00040 (Ver. 5.0-31.07.2008) 1 2 3 4 5 6 7 8 9 10 Consecu N - new Substance EU-INDEX-No.: CAS-No. Source (law, decree, guideline). Hazard/risk example of application/occurrence Remarks and comments (of 0.1% deviating consideration limit) tive No. V = prohibited (verboten) U = undesirable (unerwünscht) D = subject to declaration (deklarationspflichtig) V = prohibited RoHS from 100 ppm 7439-92-1 TRGS 905, Pb-stabilisers and pigments are subject to declaration. GefStoffV, Prohibited: Anhydrite neutral lead carbonate, lead hydrogen carbonate and lead Lead and compounds (red lead oxide, lead sulphate, lead (7446-14-2 ChemVerbotsV, cable coating, heat transfer medium for accumulators, hybrid integrated sulphate as dye pigment hydrogen carbonate, lead carbonate, lead phtalates, lead 1319-46-6 BatterieV, circuits, stabilisers in plastics, vessels and pipes for aggressive liquids, < 0.4 % in batteries 1 V N acetates, lead phosphates, lead stereates , etc) lead 598-63-0 2002/95/EG (RoHS), K3, R 1, R 3 V E F according to RoHS: Lead as alloying additive in aluminum (up to 0.4 mass%) in steel (up to 67/458/EEC pigment production, lead alloys, anti-corrosion agents (fuel additives), chromate: refer to 0.35 mass%), in copper (up to 4%), 301-04-2 76/769/EEC (89/667/EEC, 97/10/EC, 97/56/EC) soldering agent chromium (VI) -

Hexavalent Chromium (Cr VI) in Drinking Water
PUBLIC HEALTH GOALS FOR CHEMICALS IN DRINKING WATER HEXAVALENT CHROMIUM (Cr VI) July 2011 Governor of the State of California Edmund G. Brown Jr. Acting Secretary for Environmental Protection California Environmental Protection Agency Linda S. Adams Acting Director Office of Environmental Health Hazard Assessment George V. Alexeeff, Ph.D. Public Health Goal for Hexavalent Chromium (Cr VI) in Drinking Water Prepared by Pesticide and Environmental Toxicology Branch Office of Environmental Health Hazard Assessment California Environmental Protection Agency July 2011 PREFACE Drinking Water Public Health Goals Pesticide and Environmental Toxicology Branch Office of Environmental Health Hazard Assessment California Environmental Protection Agency This Public Health Goal (PHG) technical support document provides information on health effects from contaminants in drinking water. PHGs are developed for chemical contaminants based on the best available toxicological data in the scientific literature. These documents and the analyses contained in them provide estimates of the levels of contaminants in drinking water that would pose no significant health risk to individuals consuming the water on a daily basis over a lifetime. The California Safe Drinking Water Act of 1996 (Health and Safety Code, Section 116365) requires the Office of Environmental Health Hazard Assessment (OEHHA) to perform risk assessments and adopt PHGs for contaminants in drinking water based exclusively on public health considerations. The Act requires that PHGs be set in accordance with the following criteria: 1. PHGs for acutely toxic substances shall be set at levels at which no known or anticipated adverse effects on health will occur, with an adequate margin of safety. 2. PHGs for carcinogens or other substances that may cause chronic disease shall be based solely on health effects and shall be set at levels that OEHHA has determined do not pose any significant risk to health. -

A Pilot Nursery Study of the Missouri Gravel Bed System On
SUNY College of Environmental Science and Forestry Digital Commons @ ESF Dissertations and Theses Winter 12-11-2017 A PILOT NURSERY STUDY OF THE MISSOURI GRAVEL BED SYSTEM ON PHYTOREMEDIATION TREE SPECIES, POPULUS DELTOIDES X POPULUS NIGRA DN34 AND PINUS NIGRA, FOR POTENTIAL GROWTH ENHANCEMENT OVER SOIL GROWN TREES Thomas Frontera [email protected] Follow this and additional works at: https://digitalcommons.esf.edu/etds Recommended Citation Frontera, Thomas, "A PILOT NURSERY STUDY OF THE MISSOURI GRAVEL BED SYSTEM ON PHYTOREMEDIATION TREE SPECIES, POPULUS DELTOIDES X POPULUS NIGRA DN34 AND PINUS NIGRA, FOR POTENTIAL GROWTH ENHANCEMENT OVER SOIL GROWN TREES" (2017). Dissertations and Theses. 45. https://digitalcommons.esf.edu/etds/45 This Open Access Thesis is brought to you for free and open access by Digital Commons @ ESF. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of Digital Commons @ ESF. For more information, please contact [email protected], [email protected]. A PILOT NURSERY STUDY OF THE MISSOURI GRAVEL BED SYSTEM ON PHYTOREMEDIATION TREE SPECIES, POPULUS DELTOIDES X POPULUS NIGRA DN34 AND PINUS NIGRA, FOR POTENTIAL GROWTH ENHANCEMENT OVER SOIL GROWN TREES by Thomas John Frontera II A thesis submitted in partial fulfillent of the requirements for the Master of Science Degree State University of New York College of Environmental Science and Forestry Syracuse, New York May 2018 Department of Landscape Architecture Approved by: Tim Toland, Major Professor Dr. Lee Newman, Steering Committee Member Dr. Doug Johnston, Steering Committee Member and Department Chair Terry Ettinger, Thesis Examiner Dr. Jose Giner, Examining Committee Chair S. Scott Shannon, Dean, The Graduate School © 2018 Copyright T.J. -

C:\Documents and Settings\Alan Smithee\My Documents\MOTM
Itkx1//7Lhmdq`knesgdLnmsg9Rshbgshsd Our ongoing search for new minerals to feature finds us scouring the more than forty separate shows that comprise the Tucson Gem & Mineral show every year, looking for large lots of interesting and attractive minerals. The search is rewarded when we make a new contact and find something especially vibrant like this month’s combination of lavender stichtite in green serpentinite! OGXRHB@K OQNODQSHDR Chemistry: Mg6Cr2(CO3)(OH)16A4H2O Basic Hydrous Magnesium Chromium Carbonate (Hydrous Magnesium Chromium Carbonate Hydroxide) Class: Carbonates Subclass: Carbonates with hydroxyl or halogen radicals Group: Hydrotalcite Crystal System: Trigonal Crystal Habits: Crystals rarely macroscopic; usually as crust-like aggregates in matrix; sometimes radiating, micaceous with flexible plates, and nodular with tuberous, irregular surface projections; also massive and fibrous. Color: Lavender, lilac, light violet, pink, or purplish. Luster: Waxy, greasy, sometimes pearly. Transparency: Transparent to translucent Streak: White to pale lilac Refractive Index: 1.516-1.542 Cleavage: Perfect in one direction Fracture: Uneven, brittle. Hardness: 1.5-2.0 Specific Gravity: 2.2 Luminescence: None Distinctive Features and Tests: Softness, color, crystal habits, occurrence in chromium-rich metamorphic environments, and frequent association with serpentinite (a greenish metamorphic rock). Stichtite can be confused with similarly colored sugilite [potassium sodium iron manganese aluminum lithium silicate, KNa2(Fe,Mn,Al)2Li2Si12O30]. -

Some Factors Affecting the Level and Persistence of Diphenyl Residues in Citrus Fruits
HAYWARD AND EDWARDS: DIPHENYL RESIDUES 315 Using a drum dryer, temperatures as high as ing the adaptation and use of the double-drum 70° C can be used for densifying orange and dryer. grapefruit powders without discoloration. Mono- glyceride derivatives are effective as release LITERATURE CITED agents during the densifying operation. Recon- 1. Berry, R. E., O. W. Bissett, C. J. Wagner, Jr., and stitution time is increased somewhat by the densi M. K. Veldhuis 1964. Foam-mat dried grapefruit powders. Food Technol. In press. fying operation. 2. Bissett, O. W., J. H. Tatum, C. J. Wagner, Jr., M. K. Veldhuis, R. P. Graham and A. I. Morgan, Jr. 1963. Foam-mat dried orange juice. I. Time-temperature drying studies. Food Technol. 17:92-95. Acknowledgment 3. Graham, R. P., M. R. Hart and A. I. Morgan, Jr. 1964. Foam-mat drying citrus juices. Abstracts of 24th An The authors would like to thank Dr. A. I. nual Meeting of Institute of Food Technologists, No. 29. 4. Graham, R. P., L. F. Ginnette and A. I. Morgan, Jr. Morgan, Jr., and Mr. R. P. Graham of the West 1963. U. S. Patent No. 3,093,488 Preparation of stable de hydrated products. ern Regional Research Laboratory, Albany, Cali 6. Lawler, Frank K. 1962. Foam-mat drying goes to fornia, for their advice and suggestions concern work. Food Eng. 34:68-69. 6. Sjogren, C. N. 1962. Practical facts of foam-mat. Food Eng. 34:44-47. SOME FACTORS AFFECTING THE LEVEL AND PERSISTENCE OF DIPHENYL RESIDUES IN CITRUS FRUITS F.