The Muscle Spindle 0F the Rat: Peculiarities of Motor Innervation and Ultrastructure and Effect of Increased Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

Interpretation of Sensory Information from Skeletal Muscle Receptors for External Control Milan Djilas
Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control Milan Djilas To cite this version: Milan Djilas. Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control. Automatic. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00333530 HAL Id: tel-00333530 https://tel.archives-ouvertes.fr/tel-00333530 Submitted on 23 Oct 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC T H E S E pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Formation doctorale: SYSTEMES AUTOMATIQUES ET MICROELECTRONIQUES Ecole Doctorale: INFORMATION, STRUCTURES ET SYSTEMES présentée et soutenue publiquement par Milan DJILAS le 13 octobre 2008 Titre: INTERPRETATION DES INFORMATIONS SENSORIELLES DES RECEPTEURS DU MUSCLE SQUELETTIQUE POUR LE CONTROLE EXTERNE INTERPRETATION OF SENSORY INFORMATION FROM SKELETAL MUSCLE RECEPTORS FOR EXTERNAL CONTROL JURY Jacques LEVY VEHEL Directeur de Recherches, INRIA Rapporteur -

Tissue Engineered Myelination and the Stretch Reflex Arc Sensory Circuit: Defined Medium Ormulation,F Interface Design and Microfabrication
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2009 Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium ormulation,F Interface Design And Microfabrication John Rumsey University of Central Florida Part of the Biology Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Rumsey, John, "Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium Formulation, Interface Design And Microfabrication" (2009). Electronic Theses and Dissertations, 2004-2019. 3826. https://stars.library.ucf.edu/etd/3826 TISSUE ENGINEERED MYELINATION AND THE STRETCH REFLEX ARC SENSORY CIRCUIT: DEFINED MEDIUM FORMULATION, INTERFACE DESIGN AND MICROFABRICATION by JOHN WAYNE RUMSEY B.S. University of Florida, 2001 M.S. University of Central Florida, 2004 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Burnett School of Biomedical Sciences in the College of Medicine at the University of Central Florida Orlando, Florida Fall Term 2009 Major Professor: James J. Hickman ABSTRACT The overall focus of this research project was to develop an in vitro tissue- engineered system that accurately reproduced the physiology of the sensory elements of the stretch reflex arc as well as engineer the myelination of neurons in the systems. In order to achieve this goal we hypothesized that myelinating culture systems, intrafusal muscle fibers and the sensory circuit of the stretch reflex arc could be bioengineered using serum-free medium formulations, growth substrate interface design and microfabrication technology. -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
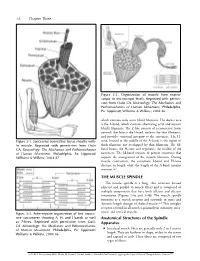
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

Muscle Physiology Dr
Muscle Physiology Dr. Ebneshahidi Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Skeletal Muscle Figure 9.2 (a) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Functions of the muscular system . 1. Locomotion . 2. Vasoconstriction and vasodilatation- constriction and dilation of blood vessel Walls are the results of smooth muscle contraction. 3. Peristalsis – wavelike motion along the digestive tract is produced by the Smooth muscle. 4. Cardiac motion . 5. Posture maintenance- contraction of skeletal muscles maintains body posture and muscle tone. 6. Heat generation – about 75% of ATP energy used in muscle contraction is released as heat. Copyright. © 2004 Pearson Education, Inc., publishing as Benjamin Cummings . Striation: only present in skeletal and cardiac muscles. Absent in smooth muscle. Nucleus: smooth and cardiac muscles are uninculcated (one nucleus per cell), skeletal muscle is multinucleated (several nuclei per cell ). Transverse tubule ( T tubule ): well developed in skeletal and cardiac muscles to transport calcium. Absent in smooth muscle. Intercalated disk: specialized intercellular junction that only occurs in cardiac muscle. Control: skeletal muscle is always under voluntary control‚ with some exceptions ( the tongue and pili arrector muscles in the dermis). smooth and cardiac muscles are under involuntary control. Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Innervation: motor unit . a) a motor nerve and a myofibril from a neuromuscular junction where gap (called synapse) occurs between the two structures. at the end of motor nerve‚ neurotransmitter (i.e. acetylcholine) is stored in synaptic vesicles which will release the neurotransmitter using exocytosis upon the stimulation of a nerve impulse. Across the synapse the surface the of myofibril contains receptors that can bind with the neurotransmitter. -

Be Able to Describe a Stretch Reflex
be able to describe a stretch reflex .(1) (2) Define muscle tone (3) be able to explain what is muscle tone (4) describe the structure , innervations and function of the muscle spindle . (5) explain what is meant by static and dynamic stretch reflex . (6) describe the spinal and supraspinal regulation of the stretch reflex . (7) describe the inverse stretch reflex and its function Stretch reflex: Whenever a muscle is stretched suddenly, excitation of the muscle spindle causes reflex contraction of the large muscle fiber (See the pictures). It is deep and monosynaptic reflex. Stretch response is produced by co-activation of alpha & gamma motor neurons. But it is maintained mainly by the tonic ( continuous) discharge of Gamma Efferent neurons. Muscle spindle: It is a sensory receptors which are distributed throughout the muscle and it sends information to nervous system about muscle length or rate of change in muscle length. - Each spindle is built around 3-12 intrafusal muscle fibers. - Muscle spindles are parallel to extrafusal muscle fibers and they are attached to them or to the tendon. Types of Intrafusal fibers Each intrafusal fiber consists of: (1) Central non-contractile area (receptor 2) Nuclear chain fibers: area). thinner and shorter 1) Nuclear bag fibers: than nuclear bag fibers (2) Peripheral contractile area. contain many nuclei in , and have one line of a dilated central area ( “ nuclei spread in a chain bag ” ) . Typically there along the receptor area are 2 nuclear bag fibers . There are 4 – 9 per spindle . nuclear chain -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Effect of Lntrafusal Muscle Mechanics on Mammalian Muscle Spindle Sensitivity’
0270.6474/85/0507-1881$02.00/O The Journal of Neurowence CopyrIght 0 Society for Neuroscience Vol. 5, No. 7, pp. 1881-1885 Printed in U.S.A. July 1985 Effect of lntrafusal Muscle Mechanics on Mammalian Muscle Spindle Sensitivity’ R. E. POPPELE*V2 AND D. c. QUICK* * Laboratory of Neurophysiology and $ Department of Anatomy, University of Minnesota, Minneapolis, Minnesota 55455 Abstract Materials and Methods Spindles were dissected free from tenuissimus muscles taken from anes- Sensitivity differences between primary and secondary thetized cats (pentobarbttal sodium, Nembutal, Abbott Laboratories, 35 mg/ endings of mammalian muscle spindles under various con- kg, or ketamine hydrochloride, Parke, Davis, 20 mg/kg). The isolated recep- ditions of stretch and fusimotor activation may be due to tor, together with about 1 cm of nerve, was mounted in a small chamber by differences in their respective mechanoelectric transducers tying each pole (near the capsule sleeve) to a small tungsten wire shaft or to mechanical properties of the intrafusal muscle support- connected to a servo-controlled Ling vibrator (model 108). The chamber was ing those endings. This study of isolated cat muscle spindles continuously perfused with oxygenated, modified Krebs’ solution (Poppele et al., 1979). The nerve was drawn onto a pair of electrodes in an adjacent examines the strain in individual intrafusal muscle fibers chamber containtng a high density fluorocarbon compound (FC-80, 3M Co.). resulting from stretch and fusimotor stimulation. The degree The entire assembly was mounted on a Zeiss photomicroscope equipped of local stretch occurring at the sensory endings under these with tine camera and Nomarski optics (see Poppele et al., 1979, and Poppele conditions was measured. -

Titin N2A Domain and Its Interactions at the Sarcomere
International Journal of Molecular Sciences Review Titin N2A Domain and Its Interactions at the Sarcomere Adeleye O. Adewale and Young-Hoon Ahn * Department of Chemistry, Wayne State University, Detroit, MI 48202, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-(313)-577-1384 Abstract: Titin is a giant protein in the sarcomere that plays an essential role in muscle contraction with actin and myosin filaments. However, its utility goes beyond mechanical functions, extending to versatile and complex roles in sarcomere organization and maintenance, passive force, mechanosens- ing, and signaling. Titin’s multiple functions are in part attributed to its large size and modular structures that interact with a myriad of protein partners. Among titin’s domains, the N2A element is one of titin’s unique segments that contributes to titin’s functions in compliance, contraction, structural stability, and signaling via protein–protein interactions with actin filament, chaperones, stress-sensing proteins, and proteases. Considering the significance of N2A, this review highlights structural conformations of N2A, its predisposition for protein–protein interactions, and its multiple interacting protein partners that allow the modulation of titin’s biological effects. Lastly, the nature of N2A for interactions with chaperones and proteases is included, presenting it as an important node that impacts titin’s structural and functional integrity. Keywords: titin; N2A domain; protein–protein interaction 1. Introduction Citation: Adewale, A.O.; Ahn, Y.-H. The complexity of striated muscle is defined by the intricate organization of its com- Titin N2A Domain and Its ponents [1]. The involuntary cardiac and voluntary skeletal muscles are the primary types Interactions at the Sarcomere. -

Malak Shalfawi Noor Adnan Lina Abdelhadi Fasial Mohammad
9 Noor Adnan Malak Shalfawi Lina abdelhadi Fasial Mohammad 0 Motor system – Motor of the spinal cord Before we start talking about the motor function of the spinal cord, let’s first take a quick look at the motor system and the incredible connections taking place inside it: [please refer to the pictures for better understanding] 1- Motor command For any motor function (movement) to occur, the nervous system has motor command that comes from the cerebral motor cortex to the spinal cord and these descending tracts (corticospinal) are called also pyramidal tracts and that’s because they pass through the pyramids of medulla oblongata. The neuronal fibers coming from the cortex ending in the spinal cord are considered upper motor neurons, while the neuronal fibers going out from the spinal cord to reach the muscles are called lower motor neurons. There are other origins for the motor commands such as the brain stem and the red nucleus that send neuronal fibers to the spinal cord in order to control the activity of the muscles. 2- Motor command intension At the same time, there are some tracts going from the cortex to the cerebellum through the brain stem like the corticopontocerebellar, corticoreticulocerebellar, corticolivarycerebellar tracts. And these tracts are telling the cerebellum about the intended movements. (= the movements we want to do) 3-motor command monitor/ feedback system a- Inside the muscles we have receptors (muscle spindles/ stretch receptors, Golgi tendon organs) that are connected to sensory (afferent) neuronal fibers that goes to the spinal cord relay nuclei. 1 | P a g e b- From the spinal cord they go to the cerebellum through ventral and dorsal spinocerebellar tracts to tell the cerebellum what is exactly happening down at the level of the muscles.