Conversion Factors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2 Amount and Concentration: Making and Diluting Solutions 2 Amount and Concentration; Making and Diluting Solutions
Essential Maths for Medics and Vets Reference Materials Module 2. Amount and Concentration. 2 Amount and concentration: making and diluting solutions 2 Amount and concentration; making and diluting solutions.........................................................1 2A Rationale.............................................................................................................................1 2B Distinguishing between amount and concentration, g and %w/v..........................................1 2C Distinguishing between amount and concentration, moles and molar...................................2 2D Practice converting g/L to M and vice versa........................................................................3 2E Diluting Solutions ...............................................................................................................5 2F Practice calculating dilutions ...............................................................................................6 Summary of learning objectives................................................................................................7 2A Rationale Biological and biochemical investigations rely completely upon being able to detect the concentration of a variety of substances. For example, in diabetics it is important to know the concentration of glucose in the blood and you may also need to be able to calculate how much insulin would need to be dissolved in a certain volume of saline so as to give the right amount in a 1ml injection volume. It is also vitally -
Unit 1 an Introduction to Fluids
UNIT 1 AN INTRODUCTION TO FLUIDS If the rock is not too heavy, you might just _ it up and carry it. When a person moves a rock, the energy to move the rock is suppl ied by the 6. When a rock fall s from a cliff, then it is gravity that is supplying the _ For a fluid to flow, there (must/need not) be a source of energy. 8. When a pump is forcing fluids through a well-bore, the source of energy for flow is the _ 9. The source of energy for a flowing well is re servoir pressure. A flowing well produces because of the of the fluids in the reservoir. 10. Any substance that can flow and that has no definite shape is a fluid. The oil in this tank (has a definite shape/assumes the shape of the tank), Gas (flows/does not flow) and has (a definite/ an indefinite) shape. 12. Anything that flows and has an indefinite shape is a (liquid/ gas/fluid), 15. The oil and gas found in a reservoir are made up of hydrogen atoms and carbon atoms. Hydrocarbons are substances made up only of atoms of ______ and atoms of _ 17. Water is made up of hydrogen atoms and oxygen atoms (H2O)· 18. Different oils and petroleum gases are made up of different combinations of hydrogen and carbon atoms. 21. A substance, water for example, can exist as a gas, a liquid, or a solid. We can change the liquid water to a gas by adding even more 23 Molecules have attractive forces which hold them together. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Measuring in Metric Units BEFORE Now WHY? You Used Metric Units
Measuring in Metric Units BEFORE Now WHY? You used metric units. You’ll measure and estimate So you can estimate the mass using metric units. of a bike, as in Ex. 20. Themetric system is a decimal system of measurement. The metric Word Watch system has units for length, mass, and capacity. metric system, p. 80 Length Themeter (m) is the basic unit of length in the metric system. length: meter, millimeter, centimeter, kilometer, Three other metric units of length are themillimeter (mm) , p. 80 centimeter (cm) , andkilometer (km) . mass: gram, milligram, kilogram, p. 81 You can use the following benchmarks to estimate length. capacity: liter, milliliter, kiloliter, p. 82 1 millimeter 1 centimeter 1 meter thickness of width of a large height of the a dime paper clip back of a chair 1 kilometer combined length of 9 football fields EXAMPLE 1 Using Metric Units of Length Estimate the length of the bandage by imagining paper clips laid next to it. Then measure the bandage with a metric ruler to check your estimate. 1 Estimate using paper clips. About 5 large paper clips fit next to the bandage, so it is about 5 centimeters long. ch O at ut! W 2 Measure using a ruler. A typical metric ruler allows you to measure Each centimeter is divided only to the nearest tenth of into tenths, so the bandage cm 12345 a centimeter. is 4.8 centimeters long. 80 Chapter 2 Decimal Operations Mass Mass is the amount of matter that an object has. The gram (g) is the basic metric unit of mass. -

SI and CGS Units in Electromagnetism
SI and CGS Units in Electromagnetism Jim Napolitano January 7, 2010 These notes are meant to accompany the course Electromagnetic Theory for the Spring 2010 term at RPI. The course will use CGS units, as does our textbook Classical Electro- dynamics, 2nd Ed. by Hans Ohanian. Up to this point, however, most students have used the International System of Units (SI, also known as MKSA) for mechanics, electricity and magnetism. (I believe it is easy to argue that CGS is more appropriate for teaching elec- tromagnetism to physics students.) These notes are meant to smooth the transition, and to augment the discussion in Appendix 2 of your textbook. The base units1 for mechanics in SI are the meter, kilogram, and second (i.e. \MKS") whereas in CGS they are the centimeter, gram, and second. Conversion between these base units and all the derived units are quite simply given by an appropriate power of 10. For electromagnetism, SI adds a new base unit, the Ampere (\A"). This leads to a world of complications when converting between SI and CGS. Many of these complications are philosophical, but this note does not discuss such issues. For a good, if a bit flippant, on- line discussion see http://info.ee.surrey.ac.uk/Workshop/advice/coils/unit systems/; for a more scholarly article, see \On Electric and Magnetic Units and Dimensions", by R. T. Birge, The American Physics Teacher, 2(1934)41. Electromagnetism: A Preview Electricity and magnetism are not separate phenomena. They are different manifestations of the same phenomenon, called electromagnetism. One requires the application of special relativity to see how electricity and magnetism are united. -
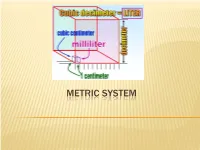
Metric System.Pdf
METRIC SYSTEM THE METRIC SYSTEM The metric system is much easier. All metric units are related by factors of 10. Nearly the entire world (95%), except the United States, now uses the metric system. Metric is used exclusively in science. Because the metric system uses units related by factors of ten and the types of units (distance, area, volume, mass) are simply-related, performing calculations with the metric system is much easier. METRIC CHART Prefix Symbol Factor Number Factor Word Kilo K 1,000 Thousand Hecto H 100 Hundred Deca Dk 10 Ten Base Unit Meter, gram, liter 1 One Deci D 0.1 Tenth Centi C 0.01 Hundredth Milli M 0.001 Thousandth The metric system has three units or bases. Meter – the basic unit used to measure length Gram – the basic unit used to measure weight Liter – the basic unit used to measure liquid capacity (think 2 Liter cokes!) The United States, Liberia and Burma (countries in black) have stuck with using the Imperial System of measurement. You can think of “the metric system” as a nickname for the International System of Units, or SI. HOW TO REMEMBER THE PREFIXES Kids Kilo Have Hecto Dropped Deca Over base unit (gram, liter, meter) Dead Deci Converting Centi Metrics Milli Large Units – Kilo (1000), Hecto (100), Deca (10) Small Units – Deci (0.1), Centi (0.01), Milli (0.001) Because you are dealing with multiples of ten, you do not have to calculate anything. All you have to do is move the decimal point, but you need to understand what you are doing when you move the decimal point. -

Water System Operator's Guide
MATH REVIEW For a rectangular tank: Most math problems a water treatment plant To find the capacity of a rectangular or square operator solves requires plugging numbers into tank: formulas and calculating the answer. When working with formulas, here are some simple Multiply length (L) by width (W) to get area (A). rules to follow. • Work from left to right. Multiply area by height (H) to get volume (V). • Do anything in parenthesis first. Multiply volume by 7.48 gallons per cubic foot to get capacity (C). • Do multiplication and division in the numerator (above the line) and in the A = L x W denominator (below the line), then do V = A x H addition and subtraction in the numerator C = V x 7.48 and denominator. • Divide the numerator by the denominator Find the capacity of a rectangular tank 15 feet (ft) last. long, 12 ft wide, and 10 ft high: Volume A = 15 ft x 12 ft = 180 square feet (ft2) The volume of a tank in cubic feet is equal to V = 180 ft2 x 10 ft = 1,800 cubic feet (ft3) the tank area multiplied by the tank height. The C = 1,800 ft3 x 7.48 gal/ft3 =13,464 gal capacity in gallons is equal to the volume in cubic feet multiplied by 7.48 gallons per cubic foot. For a circular tank: Area (A) = (3.14) x diameter squared (D2) / divided by 4 Volume (V) = A x H Capacity (C) = V x 7.48 gal/ft3 A = [ x (D2)/4] V = A x H C = V x 7.48 49 Find the capacity of a circular tank with a For an oval tank: diameter of 15 ft and a height of 12 ft: To find the gallons in an oval tank: A = [3.14 x (15 ft2)/4] = 177 ft2 Multiply the height by width by (3.14) divided by 4 to get the area of the oval. -

Metric System Conversion Factors1 J
AGR39 Metric System Conversion Factors1 J. Bryan Unruh, Barry J. Brecke, and Ramon G. Leon-Gonzalez2 Area Equivalents 1 Hectare (ha) 2 1 Acre (A) = 10,000 square meters (m ) 2 = 100 are (a) = 43,560 square feet (ft ) = 2.471 acres (A) = 4,840 square yards (yd2) = 0.405 hectares (ha) 1 Square Foot (ft) = 160 square rods (rd2) 2 = 4,047 square meters (m2) = 144 square inches (in ) = 929.03 square centimeters (cm2) 2 1 Acre-inch (ac-in) = 0.0929 square meters (m ) 3 = 102.8 cubic meters (m ) 1 Square Mile (mi) = 27,154 gallons, US (gal) 2 = 3,630 cubic feet (ft3) = 27,878,400 square feet (ft ) = 3,097,600 square yards (yd2) 2 1 Are (a) = 640 square acres (A ) = 2,589,988.11 square meters (m2) = 100 square meters (m2) 2 = 119.6 square yards (yd ) 1 Square Rod (rd) = 0.025 acre (A) = 39,204 square inches (in2) = 272.25 square feet (ft2) 1 Cubic Foot (ft) 2 3 = 30.25 square yards (yds ) = 1,728 cubic inches (in ) = 25.3 square meters (m2) = 0.037 cubic yards (yds3) 3 = 0.02832 cubic meters (cm ) 1 Square Yard (yd) = 28,320 cubic centimeters (cm3) = 9 square feet (ft2) 2 1 Cubic Yard (yd) = 0.836 square meters (m ) = 27 cubic feet (ft3) = 0.764 cubic meters (m3) 1. This document is AGR39, one of a series of the Environmental Horticulture Department, UF/IFAS Extension. Original publication date November 1993. Revised December 2014. Reviewed December 2017. Visit the EDIS website at http://edis.ifas.ufl.edu. -
Manual of Style
Manual Of Style 1 MANUAL OF STYLE TABLE OF CONTENTS CHAPTER 1 GENERAL PROVISIONS ...............3 101.0 Scope .............................................. 3 102.0 Codes and Standards..................... 3 103.0 Code Division.................................. 3 104.0 Table of Contents ........................... 4 CHAPTER 2 ADMINISTRATION .........................6 201.0 Administration ................................. 6 202.0 Chapter 2 Definitions ...................... 6 203.0 Referenced Standards Table ......... 6 204.0 Individual Chapter Administrative Text ......................... 6 205.0 Appendices ..................................... 7 206.0 Installation Standards ..................... 7 207.0 Extract Guidelines .......................... 7 208.0 Index ............................................... 9 CHAPTER 3 TECHNICAL STYLE .................... 10 301.0 Technical Style ............................. 10 302.0 Technical Rules ............................ 10 Table 302.3 Possible Unenforceable and Vague Terms ................................ 10 303.0 Health and Safety ........................ 11 304.0 Rules for Mandatory Documents .................................... 11 305.0 Writing Mandatory Requirements ............................... 12 Table 305.0 Typical Mandatory Terms............. 12 CHAPTER 4 EDITORIAL STYLE ..................... 14 401.0 Editorial Style ................................ 14 402.0 Definitions ...................................... 14 403.0 Units of Measure ............................ 15 404.0 Punctuation -

Estimating the Board Foot to Cubic Foot Ratio
United States Department of Agriculture Estimating the Forest Service Forest Board Foot to Products Laboratory Cubic Foot Ratio Research Paper FPL-RP-616 Steve Verrill Victoria L. Herian Henry Spelter Abstract Contents Certain issues in recent softwood lumber trade negotiations Page have centered on the method for converting estimates of 1 Introduction .................................................................... 1 timber volumes reported in cubic meters to board feet. Such conversions depend on many factors; three of the most im- 2 The F3 × F2 × F1 Model.................................................. 2 portant of these are log length, diameter, and taper. Average log diameters vary by region and have declined in the west- 3 The F1 Factor.................................................................. 2 ern United States due to the growing scarcity of large diame- ter, old-growth trees. Such a systematic reduction in size in 4 F3 × F2............................................................................. 3 the log population affects volume conversions from cubic units to board feet, which makes traditional rule of thumb 5 Applying the F3 × F2 × F1 Model to a Population conversion factors antiquated. In this paper we present an of West Coast Logs ........................................................ 3 improved empirical method for performing cubic volume to board foot conversions. 6 Smoothing the F3 × F2 Surface....................................... 4 Keywords: Scribner scaling, diameter, length, taper, 7 Optimal Smoothing -

A General Introduction on Metrology and Traceability
A general introduction on metrology and traceability Paul Brewer LNG metrology workshop 15th June 2016 National Physical Laboratory • Develop and disseminate UK’s measurement standards, ensure international acceptance • Knowledge transfer and advice between industry, government and academia • Support Industry, trade, regulation, legislation, quality of life, science and innovation industrial environment energy Gas and Particle particles Metrology The Fundamentals of Metrology • What is metrology and what is it for? • What is an NMI and what is it for? • What is the mole and what is it for? What is ‘Metrology’? . Metrology is “the science of measurement, embracing both experimental and theoretical determinations at any level of uncertainty in any field of science and technology.” . Almost all of science and industry involves making and interpreting measurement – why is metrology special? The Proclamation Regarding Weights and Measures, 1556 by Ford Madox Brown (1889) The electrochemical characteristics of platinum phthalocyanine . Quantitative conclusions inferred; but what was the accuracy, repeatability, reproducibility and uncertainty of these measurements? . Would this have affected the conclusions? Metrology’s main activities . The definition of internationally accepted units of measurement, e.g. the kilogram . The realisation of units of measurement by scientific methods . The establishment of (metrological) traceability chains by disseminating and documenting the value and accuracy of a measurement . Traceability implies the calculation of an associated measurement uncertainty . These activities may be fundamental (scientific) or applied (practical, industrial, legal) International vocabulary of metrology The Results of Metrology . Generates systems and frameworks for quantification and through these underpins consistency and assurance in all measurement . Gives a quantified level of confidence in the measurement through an uncertainty statement .