Yellow Fever
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hoosiers and the American Story Chapter 3
3 Pioneers and Politics “At this time was the expression first used ‘Root pig, or die.’ We rooted and lived and father said if we could only make a little and lay it out in land while land was only $1.25 an acre we would be making money fast.” — Andrew TenBrook, 1889 The pioneers who settled in Indiana had to work England states. Southerners tended to settle mostly in hard to feed, house, and clothe their families. Every- southern Indiana; the Mid-Atlantic people in central thing had to be built and made from scratch. They Indiana; the New Englanders in the northern regions. had to do as the pioneer Andrew TenBrook describes There were exceptions. Some New Englanders did above, “Root pig, or die.” This phrase, a common one settle in southern Indiana, for example. during the pioneer period, means one must work hard Pioneers filled up Indiana from south to north or suffer the consequences, and in the Indiana wilder- like a glass of water fills from bottom to top. The ness those consequences could be hunger. Luckily, the southerners came first, making homes along the frontier was a place of abundance, the land was rich, Ohio, Whitewater, and Wabash Rivers. By the 1820s the forests and rivers bountiful, and the pioneers people were moving to central Indiana, by the 1830s to knew how to gather nuts, plants, and fruits from the northern regions. The presence of Indians in the north forest; sow and reap crops; and profit when there and more difficult access delayed settlement there. -

Dengue Fact Sheet
State of California California Department of Public Health Health and Human Services Agency Division of Communicable Disease Control Dengue Fact Sheet What is dengue? Dengue is a disease caused by any one of four closely related dengue viruses (DENV- 1, DENV-2, DENV-3, and DENV-4). Where does dengue occur? Dengue occurs in many tropical and sub-tropical areas of the world, particularly sub- Saharan Africa, the Middle East, Southeast Asia, and Central and South America. With the exception of Mexico, Puerto Rico, small areas in southern Texas and southern Florida, and some regions of Hawaii, dengue transmission does not occur in North America. Worldwide there are an estimated 50 to 100 million cases of dengue per year. How do people get dengue? People get dengue from the bite of an infected mosquito. The mosquito becomes infected when it bites a person who has dengue virus in their blood. It takes a week or more for the dengue organisms to mature in the mosquito; then the mosquito can transmit the virus to another person when it bites. Dengue is transmitted principally by Aedes aegypti (yellow fever mosquito) and Aedes albopictus (Asian tiger mosquito). These mosquitoes are not native to California, but infestations have been identified in multiple counties in California. Dengue virus cannot be transmitted from person to person. What are the symptoms of dengue? There are two types of illness that can result from infection with a dengue virus: dengue and severe dengue. The main symptoms of dengue are high fever, severe headache, severe pain behind the eyes, joint pain, muscle and bone pain, rash, bruising, and may include mild bleeding from the nose or mouth. -

Dengue and Yellow Fever
GBL42 11/27/03 4:02 PM Page 262 CHAPTER 42 Dengue and Yellow Fever Dengue, 262 Yellow fever, 265 Further reading, 266 While the most important viral haemorrhagic tor (Aedes aegypti) as well as reinfestation of this fevers numerically (dengue and yellow fever) are insect into Central and South America (it was transmitted exclusively by arthropods, other largely eradicated in the 1960s). Other factors arboviral haemorrhagic fevers (Crimean– include intercontinental transport of car tyres Congo and Rift Valley fevers) can also be trans- containing Aedes albopictus eggs, overcrowding mitted directly by body fluids. A third group of of refugee and urban populations and increasing haemorrhagic fever viruses (Lassa, Ebola, Mar- human travel. In hyperendemic areas of Asia, burg) are only transmitted directly, and are not disease is seen mainly in children. transmitted by arthropods at all. The directly Aedes mosquitoes are ‘peri-domestic’: they transmissible viral haemorrhagic fevers are dis- breed in collections of fresh water around the cussed in Chapter 41. house (e.g. water storage jars).They feed on hu- mans (anthrophilic), mainly by day, and feed re- peatedly on different hosts (enhancing their role Dengue as vectors). Dengue virus is numerically the most important Clinical features arbovirus infecting humans, with an estimated Dengue virus may cause a non-specific febrile 100 million cases per year and 2.5 billion people illness or asymptomatic infection, especially in at risk.There are four serotypes of dengue virus, young children. However, there are two main transmitted by Aedes mosquitoes, and it is un- clinical dengue syndromes: dengue fever (DF) usual among arboviruses in that humans are the and dengue haemorrhagic fever (DHF). -

Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on Suspicion of Infection During Business Hours
Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on suspicion of infection during business hours PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background information on the disease (see Section 2), case definitions (see Section 3 for dengue and for chikungunya), and laboratory testing (see Section 4) Forward specimens to the Florida Department of Health (DOH) Bureau of Public Health Laboratories (BPHL) for confirmatory laboratory testing (as needed) Inform local mosquito control personnel of suspected chikungunya or dengue case as soon as possible (if applicable) Inform state Arbovirus Surveillance Coordinator on suspicion of locally acquired arbovirus infection Contact provider (see Section 5A) Interview case-patient Review disease facts (see Section 2) Mode of transmission Ask about exposure to relevant risk factors (see Section 5. Case Investigation) History of travel, outdoor activities, and mosquito bites two weeks prior to onset History of febrile illness or travel for household members or other close contacts in the month prior to onset History of previous arbovirus infection or vaccination (yellow fever, Japanese encephalitis) Provide education on transmission and prevention (see Section 6) Awareness of mosquito-borne diseases Drain standing water at least weekly to stop mosquitoes from multiplying Discard items that collect water and are not being used Cover skin with clothing or Environmental Protection Agency (EPA)-registered repellent such as DEET (N,N-diethyl-meta-toluamide) Use permethrin on clothing (not skin) according to manufacturer’s directions Cover doors and windows with intact screens to keep mosquitoes out of the house Enter additional data obtained from interview into Merlin (see Section 5D) Arrange for a convalescent specimen to be taken (if necessary) Dengue/Chikungunya Guide to Surveillance and Investigation Dengue Fever/Severe Dengue/Chikungunya 1. -

Ebola Virus Disease and Clinical Care Part I: History, Transmission, and Clinical Presentation
Ebola Virus Disease and Clinical Care Part I: History, Transmission, and Clinical Presentation This lecture is on Ebola virus disease (EVD) and clinical care. This is part one of a three-part lecture on this topic. Preparing Healthcare Workers to Work in Ebola Treatment Units (ETUs) in Africa This lecture will focus on EVD in the West African setting. Ebola Virus Disease and Clinical Care: The training and information you receive in this course will Part I: History, Transmission, and Clinical not cover the use of certain interventions such as intubation Presentation or dialysis which are not available in West African Ebola Treatment Units (ETUs). You will need supplemental training This presentation is current as of December 2014. This presentation contains materials from Centers for Disease Control and to care for patients appropriately in countries where advanced Prevention (CDC), Médecins Sans Frontières (MSF), and World Health Organization (WHO). care is available. U.S. Department of Health and Human Services U.S. Department of Health and Human Services Centers for Disease Control and Prevention Centers for Disease Control and Prevention version 12.03.2014 The learning objectives for this lecture are to: Learning Objectives ▶ Describe the routes of Ebola virus transmission Describe the routes of Ebola virus transmission Explain when and how patients are infectious ▶ Explain when and how patients are infectious Describe the clinical features of patients with Ebola ▶ Describe screening criteria for Ebola virus disease Describe the clinical features of patients with Ebola (EVD) used in West Africa Explain how to identify patients with suspected ▶ Describe screening criteria for EVD used in West Africa EVD who present to the ETU ▶ Explain how to identify patients with suspected EVD who present to the ETU This presentation contains materials from CDC, MSF, and WHO 2 A number of different viruses cause viral hemorrhagic fever. -
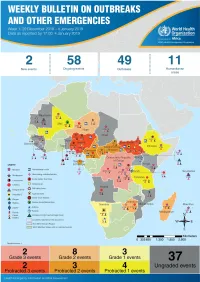
Health Emergency Information and Risk Assessment Health Emergency Information and Risk Assessment Overview
1 Health Emergency Information and Risk Assessment Health Emergency Information and Risk Assessment Overview Contents This Weekly Bulletin focuses on selected acute public health emergencies occurring in the WHO African Region. The WHO Health Emergencies Programme (WHE) is currently monitoring 60 events in the region. This week’s edition covers key ongoing events, including: 2 Overview Ebola virus disease outbreak in the Democratic Republic of the Congo 3 - 6 Ongoing events Cholera in Burundi Cholera in Cameroon 7 Summary of major Yellow fever in Nigeria. issues challenges and proposed actions For each of these events, a brief description, followed by public health measures implemented and an interpretation of the situation is provided. 8 All events currently being monitored Major issues and challenges include: The Ebola virus disease (EVD) outbreak in the Democratic Republic of the Congo (DRC) is in a critical phase as it enters its sixth month since the declaration of the outbreak. Despite the use of an effective vaccine, novel therapeutics as well as other EVD strategic interventions, the outbreak is persisting due to security challenges, pockets of community reluctance and inadequate infection prevention and control in some health facilities. Nevertheless, WHO and partners, under the government’s leadership, continue to respond to the EVD outbreak and remain committed to containing the outbreak. The Ministry of Health of Burundi has declared a new outbreak of cholera in the country. This outbreak, which is rapidly evolving, is particularly affecting people living in overcrowded areas, where sanitation conditions are precarious. Given that the risk factors for transmission of water-borne diseases are prevalent in the affected communities, there is a need to aggressively tackle this outbreak at its early stage using relevant sectors in order to avoid further spread. -

Top Tips How to Avoid Vector Borne Diseases (Eng)
Tr a a v r e i ljl s k in a g r e to a Vectors such as mosquitoes, sandflies and HOW tO AvOid ticks can transmit serious infectious diseases both in vEctOR-BORnE the WHO European Region disEAsEs – tOp tips and globally. Many of these vectors are blood sucking and transfer diseases to Take simple measures to protect yourself and your family. human beings through their bites. 1. Before you travel, be vaccinated against diseases prevalent at your destination. vaccines exist for yellow fever, Japanese encephalitis and tick- Avoid being bitten by a borne encephalitis. mosquito, sandfly or tick. 2. consult your doctor, 4–6 weeks before departure if possible, to discuss how you can protect yourself (for example, what antimalarial medicines you Be prepared. should take if malaria is endemic at your destination). 3. Wear light-coloured, long-sleeved shirts and long trousers, tucked into socks or boots, and use insect repellent on exposed skin and clothing to protect yourself from being bitten by mosquitoes, sandflies or ticks. temperature, humidity and the time of day affect the likelihood of being bitten, so know when you need extra protective clothing and insect repellent. 4. Use window screens, if available, to keep mosquitoes outside the place where you are staying. 5. sleep under an insecticide-treated bed net, requesting one if necessary, if you are staying in an area with malaria risk. 6. check your body regularly for ticks. if you find one, remove it with tweezers and apply a skin disinfectant. in tick-infested areas, examine your clothing, luggage and other belongings thoroughly before entering the place where you are staying. -

Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever
C o m p r e h e n s Dengue fever (DF) is the fastest emerging arboviral infection spread by Aedes aegypti i v mosquitoes with major public health consequences for millions of people around the e G world, and in particular the South-East Asia and Asia-Pacific Regions of the World u i Health Organization (WHO). Of the 2.5 billion people globally at risk of DF and its d e severe forms dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS) l i n South-East Asia accounts for approximately 1.3 billion or 52%. e s f As the disease spreads to new geographical areas, the frequency of the o r outbreaks has increased along with a rapidly changing disease epidemiology. In P r response to resolution of the Forty-sixth World Health Assembly urging Member e v States to strengthen national programmes for control of DF/DHF, several documents e n were developed by regional offices of WHO, including South-East Asia. t i o In 1999 the WHO Regional Office for South-East Asia published the Regional n a Comprehensive Guidelines for Guidelines for the Prevention and Control of DF/DHF. Since then new strategies and n d developments in the control of dengue fever, DHF and DSS have come to light. The C Regional Guidelines were extensively revised, updated and expanded with the focus o n Prevention and Control of on new and additional topics of current relevance to the populations of Member t r o States of the Region. They were then rechristened the Comprehensive Guidelines for l o the Prevention and Control of Dengue and Dengue Haemmorhagic Fever. -

NBER WORKING PAPER SERIES FINANCIAL DEVELOPMENT and CITY GROWTH: EVIDENCE from NORTHEASTERN AMERICAN CITIES, 1790-1870 Howard Bo
NBER WORKING PAPER SERIES FINANCIAL DEVELOPMENT AND CITY GROWTH: EVIDENCE FROM NORTHEASTERN AMERICAN CITIES, 1790-1870 Howard Bodenhorn David Cuberes Working Paper 15997 http://www.nber.org/papers/w15997 NATIONAL BUREAU OF ECONOMIC RESEARCH 1050 Massachusetts Avenue Cambridge, MA 02138 May 2010 We thank Nate Baum-Snow, Gilles Duranton, Javier Gardeazábal, Henry Overman, Francesco Serti, and seminar participants at the Urban Economics Association 2009, University of the Basque Country, and Brown University for useful comments and Michael Haines for providing digitized files of the 1790 through 1870 censuses. Adam Blott, Laura Lamontagne and Pam Bodenhorn offered very able research assistance. Cuberes acknowledges the financial support of the Ministerio de Ciencia e Innovación and FEDER funds (proyecto SEJ2007-62656) and Bodenhorn the financial support of Clemson University. The views expressed herein are those of the authors and do not necessarily reflect the views of the National Bureau of Economic Research. NBER working papers are circulated for discussion and comment purposes. They have not been peer- reviewed or been subject to the review by the NBER Board of Directors that accompanies official NBER publications. © 2010 by Howard Bodenhorn and David Cuberes. All rights reserved. Short sections of text, not to exceed two paragraphs, may be quoted without explicit permission provided that full credit, including © notice, is given to the source. Financial Development and City Growth: Evidence from Northeastern American Cities, 1790-1870 Howard Bodenhorn and David Cuberes NBER Working Paper No. 15997 May 2010 JEL No. N11,N90,R11 ABSTRACT Using cross sectional and panel techniques, we find a positive and strong correlation between financial development and subsequent city growth in the Northeastern United States between 1790 and 1870. -

Guide Yellow Fever Outbreak 2016
© World Health Organization 2016 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. -

The War of 1812
The War of 1812 Two hundred years ago Britain and the United States went to war. The conflict was a relatively minor affair, but its consequences were great, says Jeremy Black. The War of 1812 nglo-American relations, though extolled George Munger's drawing was killed attacking the Americans (see 'The Saviour since the 1940s, have often been difficult and of the unfinished Capitol of Canada', p. 13). never more so than when the two powers building in ruins after the American hopes were also disappointed in the British set fire to it in retali- Awent to war in 1812. Sometimes referred to ation for destruction at distant, snowy wastes of Russia. America had chosen to as a forgotten conflict, the War of 1812 played a major York (now Toronto) the go to war with Britain in June 1812 at a moment when role in defining relations between the two states. previous year. Britain appeared vulnerable. Napoleon's invasion of Episodes ofthe conflict are celebrated in American Russia, which followed a week later, represented, in its public myth, notably the defence of Fort McHenry in constituent army, an alliance of most of Europe. Antici- 1814, the origin of 'The Star-Spangled Banner', and pating French success. President Madison hoped that Andrew Jackson's victory outside New Orleans in 1815. Britain would be forced to concede the loss of Canada. The Americans went to war in 1812 in order to end Yet fi^om late 1812 Britain's position strengthened Britain's blockade of trade with Napoleon's France and in dramatically. -

Key Messages and Actions for Zika Prevention and Control: Guide for Schools Contents
© UNICEF/UN018982/Arcos Key Messages and Actions for Zika Prevention and Control: Guide for Schools Contents Preface 3 Introduction 4 1. Zika virus 6 Zika signs and symptoms 6 Treatment: Zika 6 2. Other Diseases Transmitted by the Aedes Mosquito 8 Chikungunya virus 8 Yellow fever virus 8 Dengue virus 8 Protect yourself and school community members from 10 mosquito bites at school and at home Prevention of mosquito borne viruses 12 3. Key actions for school administrators, teachers, and staff 14 4. Key actions for parents and community members 19 Engagement of Staff and Children 19 5. Age specific Health Education – Zika 20 5–11 years of age 20 12–16 years of age 21 A Zika checklist for students 23 6. Additional resources/tools 24 ANNEX 1 25 Preface On 1 February 2016, the World Health Organization (WHO) determined that the clusters of microcephaly and other neurological disorders constitute a Public Health Emergency of International Concern (PHEIC). WHO has developed a Strategic Response Framework (SRF) and Joint Operations Plan in response to the Zika outbreak. This guide for schools, developed by UNICEF with the support of WHO and the Centers for Disease Control and Prevention (CDC), aims to provide guidance on Zika prevention and control in the school setting to complement the Strategic Response Framework. The target audiences for this document includes Ministries of Education (national, provincial, and district level), school administrators, teachers and students, as well as program managers and policy makers from other organizations supporting education programs and systems. In addition, the guide contains information that can be adapted for students and their parents in the wider school community.