Dengue Fact Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dengue and Yellow Fever
GBL42 11/27/03 4:02 PM Page 262 CHAPTER 42 Dengue and Yellow Fever Dengue, 262 Yellow fever, 265 Further reading, 266 While the most important viral haemorrhagic tor (Aedes aegypti) as well as reinfestation of this fevers numerically (dengue and yellow fever) are insect into Central and South America (it was transmitted exclusively by arthropods, other largely eradicated in the 1960s). Other factors arboviral haemorrhagic fevers (Crimean– include intercontinental transport of car tyres Congo and Rift Valley fevers) can also be trans- containing Aedes albopictus eggs, overcrowding mitted directly by body fluids. A third group of of refugee and urban populations and increasing haemorrhagic fever viruses (Lassa, Ebola, Mar- human travel. In hyperendemic areas of Asia, burg) are only transmitted directly, and are not disease is seen mainly in children. transmitted by arthropods at all. The directly Aedes mosquitoes are ‘peri-domestic’: they transmissible viral haemorrhagic fevers are dis- breed in collections of fresh water around the cussed in Chapter 41. house (e.g. water storage jars).They feed on hu- mans (anthrophilic), mainly by day, and feed re- peatedly on different hosts (enhancing their role Dengue as vectors). Dengue virus is numerically the most important Clinical features arbovirus infecting humans, with an estimated Dengue virus may cause a non-specific febrile 100 million cases per year and 2.5 billion people illness or asymptomatic infection, especially in at risk.There are four serotypes of dengue virus, young children. However, there are two main transmitted by Aedes mosquitoes, and it is un- clinical dengue syndromes: dengue fever (DF) usual among arboviruses in that humans are the and dengue haemorrhagic fever (DHF). -

Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on Suspicion of Infection During Business Hours
Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on suspicion of infection during business hours PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background information on the disease (see Section 2), case definitions (see Section 3 for dengue and for chikungunya), and laboratory testing (see Section 4) Forward specimens to the Florida Department of Health (DOH) Bureau of Public Health Laboratories (BPHL) for confirmatory laboratory testing (as needed) Inform local mosquito control personnel of suspected chikungunya or dengue case as soon as possible (if applicable) Inform state Arbovirus Surveillance Coordinator on suspicion of locally acquired arbovirus infection Contact provider (see Section 5A) Interview case-patient Review disease facts (see Section 2) Mode of transmission Ask about exposure to relevant risk factors (see Section 5. Case Investigation) History of travel, outdoor activities, and mosquito bites two weeks prior to onset History of febrile illness or travel for household members or other close contacts in the month prior to onset History of previous arbovirus infection or vaccination (yellow fever, Japanese encephalitis) Provide education on transmission and prevention (see Section 6) Awareness of mosquito-borne diseases Drain standing water at least weekly to stop mosquitoes from multiplying Discard items that collect water and are not being used Cover skin with clothing or Environmental Protection Agency (EPA)-registered repellent such as DEET (N,N-diethyl-meta-toluamide) Use permethrin on clothing (not skin) according to manufacturer’s directions Cover doors and windows with intact screens to keep mosquitoes out of the house Enter additional data obtained from interview into Merlin (see Section 5D) Arrange for a convalescent specimen to be taken (if necessary) Dengue/Chikungunya Guide to Surveillance and Investigation Dengue Fever/Severe Dengue/Chikungunya 1. -

Ebola Virus Disease and Clinical Care Part I: History, Transmission, and Clinical Presentation
Ebola Virus Disease and Clinical Care Part I: History, Transmission, and Clinical Presentation This lecture is on Ebola virus disease (EVD) and clinical care. This is part one of a three-part lecture on this topic. Preparing Healthcare Workers to Work in Ebola Treatment Units (ETUs) in Africa This lecture will focus on EVD in the West African setting. Ebola Virus Disease and Clinical Care: The training and information you receive in this course will Part I: History, Transmission, and Clinical not cover the use of certain interventions such as intubation Presentation or dialysis which are not available in West African Ebola Treatment Units (ETUs). You will need supplemental training This presentation is current as of December 2014. This presentation contains materials from Centers for Disease Control and to care for patients appropriately in countries where advanced Prevention (CDC), Médecins Sans Frontières (MSF), and World Health Organization (WHO). care is available. U.S. Department of Health and Human Services U.S. Department of Health and Human Services Centers for Disease Control and Prevention Centers for Disease Control and Prevention version 12.03.2014 The learning objectives for this lecture are to: Learning Objectives ▶ Describe the routes of Ebola virus transmission Describe the routes of Ebola virus transmission Explain when and how patients are infectious ▶ Explain when and how patients are infectious Describe the clinical features of patients with Ebola ▶ Describe screening criteria for Ebola virus disease Describe the clinical features of patients with Ebola (EVD) used in West Africa Explain how to identify patients with suspected ▶ Describe screening criteria for EVD used in West Africa EVD who present to the ETU ▶ Explain how to identify patients with suspected EVD who present to the ETU This presentation contains materials from CDC, MSF, and WHO 2 A number of different viruses cause viral hemorrhagic fever. -
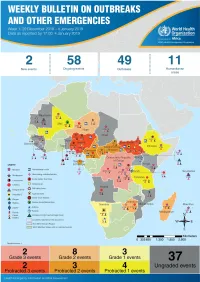
Health Emergency Information and Risk Assessment Health Emergency Information and Risk Assessment Overview
1 Health Emergency Information and Risk Assessment Health Emergency Information and Risk Assessment Overview Contents This Weekly Bulletin focuses on selected acute public health emergencies occurring in the WHO African Region. The WHO Health Emergencies Programme (WHE) is currently monitoring 60 events in the region. This week’s edition covers key ongoing events, including: 2 Overview Ebola virus disease outbreak in the Democratic Republic of the Congo 3 - 6 Ongoing events Cholera in Burundi Cholera in Cameroon 7 Summary of major Yellow fever in Nigeria. issues challenges and proposed actions For each of these events, a brief description, followed by public health measures implemented and an interpretation of the situation is provided. 8 All events currently being monitored Major issues and challenges include: The Ebola virus disease (EVD) outbreak in the Democratic Republic of the Congo (DRC) is in a critical phase as it enters its sixth month since the declaration of the outbreak. Despite the use of an effective vaccine, novel therapeutics as well as other EVD strategic interventions, the outbreak is persisting due to security challenges, pockets of community reluctance and inadequate infection prevention and control in some health facilities. Nevertheless, WHO and partners, under the government’s leadership, continue to respond to the EVD outbreak and remain committed to containing the outbreak. The Ministry of Health of Burundi has declared a new outbreak of cholera in the country. This outbreak, which is rapidly evolving, is particularly affecting people living in overcrowded areas, where sanitation conditions are precarious. Given that the risk factors for transmission of water-borne diseases are prevalent in the affected communities, there is a need to aggressively tackle this outbreak at its early stage using relevant sectors in order to avoid further spread. -

Key Messages and Actions for Zika Prevention and Control: Guide for Schools Contents
© UNICEF/UN018982/Arcos Key Messages and Actions for Zika Prevention and Control: Guide for Schools Contents Preface 3 Introduction 4 1. Zika virus 6 Zika signs and symptoms 6 Treatment: Zika 6 2. Other Diseases Transmitted by the Aedes Mosquito 8 Chikungunya virus 8 Yellow fever virus 8 Dengue virus 8 Protect yourself and school community members from 10 mosquito bites at school and at home Prevention of mosquito borne viruses 12 3. Key actions for school administrators, teachers, and staff 14 4. Key actions for parents and community members 19 Engagement of Staff and Children 19 5. Age specific Health Education – Zika 20 5–11 years of age 20 12–16 years of age 21 A Zika checklist for students 23 6. Additional resources/tools 24 ANNEX 1 25 Preface On 1 February 2016, the World Health Organization (WHO) determined that the clusters of microcephaly and other neurological disorders constitute a Public Health Emergency of International Concern (PHEIC). WHO has developed a Strategic Response Framework (SRF) and Joint Operations Plan in response to the Zika outbreak. This guide for schools, developed by UNICEF with the support of WHO and the Centers for Disease Control and Prevention (CDC), aims to provide guidance on Zika prevention and control in the school setting to complement the Strategic Response Framework. The target audiences for this document includes Ministries of Education (national, provincial, and district level), school administrators, teachers and students, as well as program managers and policy makers from other organizations supporting education programs and systems. In addition, the guide contains information that can be adapted for students and their parents in the wider school community. -

Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept
pathogens Article Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept Ravendra P. Chauhan 1 , Zelalem G. Dessie 2,3 , Ayman Noreddin 4,5 and Mohamed E. El Zowalaty 4,6,7,* 1 School of Laboratory Medicine and Medical Sciences, College of Health Sciences, University of KwaZulu-Natal, Durban 4001, South Africa; [email protected] 2 School of Mathematics, Statistics and Computer Science, University of KwaZulu-Natal, Durban 4001, South Africa; [email protected] 3 Department of Statistics, College of Science, Bahir Dar University, Bahir Dar 6000, Ethiopia 4 Infectious Diseases and Anti-Infective Therapy Research Group, Sharjah Medical Research Institute and College of Pharmacy, University of Sharjah, Sharjah 27272, UAE; [email protected] 5 Department of Medicine, School of Medicine, University of California, Irvine, CA 92868, USA 6 Zoonosis Science Center, Department of Medical Biochemistry and Microbiology, Uppsala University, SE 75185 Uppsala, Sweden 7 Division of Virology, Department of Infectious Diseases and St. Jude Center of Excellence for Influenza Research and Surveillance (CEIRS), St Jude Children Research Hospital, Memphis, TN 38105, USA * Correspondence: [email protected] Received: 17 February 2020; Accepted: 7 April 2020; Published: 20 April 2020 Abstract: Emerging and re-emerging viral diseases are of great public health concern. The recent emergence of Severe Acute Respiratory Syndrome (SARS) related coronavirus (SARS-CoV-2) in December 2019 in China, which causes COVID-19 disease in humans, and its current spread to several countries, leading to the first pandemic in history to be caused by a coronavirus, highlights the significance of zoonotic viral diseases. -

Vaccine Prior Authorization Change Effective January 1, 2020
Prior Authorization Change Effective January 1, 2020 Apple Health (Medicaid) Effective January 1, 2020, requirements for Prior Authorization with Molina Healthcare of Washington will change for CPT codes 90675 (Rabies vaccine, for intramuscular use), 90691 (Typhoid vaccine, Vi capsular polysaccharide (ViCPs), for intramuscular use), and 90717 (Yellow fever vaccine, live, for subcutaneous use). This change affects Apple Health (Medicaid) members and does not apply to Marketplace members. Vaccines are covered only when listed on the CDC immunization schedule for adults and children in the United States. Vaccines recommended or required for the sole purpose of international travel are not covered. Additionally, pre-exposure rabies vaccine is not covered. Post-exposure treatment administration of rabies vaccines is covered, if medically necessary. Previously, CPT codes 90675, 90691 and 90717 did not require Prior Authorization. Molina will require submission of a request for both participating and non-participating providers for claims submitted for any place of service (excluding emergency services). Please submit an invoice detailing cost along with supporting documentation at the time of the Prior Authorization request. As always, clinical notes are required for review and approval of your authorization request. Submitting the clinical notes along with the Prior Authorization request is recommended to receive a timely and accurate decision. If prior authorization is required for a requested service, please fax your authorization requests to Molina at (800) 767- 7188. Forms: • For our prior authorization forms, please see our provider website at: MolinaHealthcare.com/providers/wa/medicaid/forms/PDF/mhwa-guide-request-form-medicaid-2019.pdf As always our goal is to provide you with excellent customer service. -

Zika Virus Infection 7 May 2015
Epidemiological Alert Zika virus infection 7 May 2015 The Pan American Health Organization (PAHO) / World Health Organization (WHO) recommends its Member States establish and maintain the capacity for Zika virus infection detection, clinical management and an effective public communication strategy to reduce the presence of the mosquito that transmits this disease, particularly in areas where the vector is present. Situation summary Zika virus infection The Zika virus was first isolated in 1947 in Zika Forest (Uganda), in a Rhesus monkey during a study of the This is a disease caused by the Zika virus transmission of wild yellow fever. It was first isolated in (ZIKAV), an arbovirus the flavivirus genus humans in 1952 (Uganda, Tanzania).1,2 In 1968 the virus (family Flaviviridae), very close phylogenetically to viruses such as was detected in human samples in Nigeria. 3,4 dengue, yellow fever, Japanese encephalitis, or West Nile virus. In 2007 the first major outbreak of Zika virus fever occurred on the island of Yap (Micronesia) where 185 The Zika virus is transmitted by mosquitoes suspected cases were reported, of which 49 were of the genus Aedes, in urban areas (A. confirmed and 59 were considered probable. The aegypti) as well as in the wild. outbreak lasted 13 weeks (April to July). The probable After an infected mosquito bite, the vector was identified as being Aedes hensilii, however disease symptoms usually appear the presence of the virus in the mosquito could not be following an incubation period of three determined. to twelve days. The infection may present itself as Subsequently an outbreak in French Polynesia, which asymptomatic or with a moderate began at the end of October 2013. -

Yellow Fever: Integrating Current Knowledge with Technological Innovations to Identify Strategies for Controlling a Re-Emerging Virus
viruses Review Yellow Fever: Integrating Current Knowledge with Technological Innovations to Identify Strategies for Controlling a Re-Emerging Virus 1, 1, 2, 3 Robin D.V. Kleinert y , Eduardo Montoya-Diaz y, Tanvi Khera y , Kathrin Welsch , Birthe Tegtmeyer 4 , Sebastian Hoehl 5 , Sandra Ciesek 5 and Richard J.P. Brown 1,* 1 Division of Veterinary Medicine, Paul-Ehrlich-Institute, 63225 Langen, Germany; [email protected] (R.D.V.K.); [email protected] (E.M.-D.) 2 Department of Gastroenterology and Hepatology, Faculty of Medicine, University Hospital Essen, University of Duisburg-Essen, 45147 Essen, Germany; [email protected] 3 University of Veterinary Medicine Hannover, 30559 Hannover, Germany; [email protected] 4 Institute for Experimental Virology, TWINCORE, Centre for Experimental and Clinical Infection Research; a joint venture between the Medical School Hannover (MHH) and the Helmholtz Centre for Infection Research (HZI), 30625 Hannover, Germany; [email protected] 5 Institute of Medical Virology, University Hospital Frankfurt, 60596 Frankfurt am Main, Germany; [email protected] (S.H.), [email protected] (S.C.) * Correspondence: [email protected]; Tel.: +49-6103-77-7441 These authors contributed equally to the work. y Received: 30 August 2019; Accepted: 11 October 2019; Published: 17 October 2019 Abstract: Yellow fever virus (YFV) represents a re-emerging zoonotic pathogen, transmitted by mosquito vectors to humans from primate reservoirs. Sporadic outbreaks of YFV occur in endemic tropical regions, causing a viral hemorrhagic fever (VHF) associated with high mortality rates. Despite a highly effective vaccine, no antiviral treatments currently exist. Therefore, YFV represents a neglected tropical disease and is chronically understudied, with many aspects of YFV biology incompletely defined including host range, host–virus interactions and correlates of host immunity and pathogenicity. -

West Nile Virus Infection Lineages by Including Koutango Virus, a Related Virus That America
West Nile Virus Importance West Nile virus (WNV) is a mosquito-borne virus that circulates among birds, Infection but can also affect other species, particularly humans and horses. Many WNV strains are thought to be maintained in Africa; however, migrating birds carry these viruses to other continents each year, and some strains have become established outside West Nile Fever, Africa. At one time, the distribution of WNV was limited to the Eastern Hemisphere, and it was infrequently associated with serious illness. Clinical cases usually occurred West Nile Neuroinvasive Disease, sporadically in humans and horses, or as relatively small epidemics in rural areas. West Nile Disease, Most human infections were asymptomatic, and if symptoms occurred, they were Near Eastern Equine Encephalitis, typically mild and flu-like. Severe illnesses, characterized by neurological signs, Lordige seemed to be uncommon in most outbreaks. Birds appeared to be unaffected throughout the Eastern Hemisphere, possibly because they had become resistant to the virus through repeated exposure. Since the 1990s, this picture has changed, and WNV has emerged as a significant Last Updated: August 2013 human and veterinary pathogen in the Americas, Europe, the Middle East and other areas. Severe outbreaks, with an elevated case fatality rate, were initially reported in Algeria, Romania, Morocco, Tunisia, Italy, Russia and Israel between 1994 and 1999. While approximately 80% of the people infected with these strains were still asymptomatic, 20% had flu-like signs, and a small but significant percentage (<1%) developed neurological disease. One of these virulent viruses entered the U.S. in 1999. Despite control efforts, it became established in much of North America, and spread to Central and South America and the Caribbean. -

Seoul Orthohantavirus in Wild Black Rats, Senegal, 2012–2013 Moussa M
DISPATCHES Seoul Orthohantavirus in Wild Black Rats, Senegal, 2012–2013 Moussa M. Diagne, Idrissa Dieng, Laurent Granjon, Héloïse Lucaccioni, Abdourahmane Sow, Oumar Ndiaye, Martin Faye, Khalilou Bâ, Yamar Bâ, Mamoudou Diallo, Oumar Faye, Jean-Marc Duplantier, Mawlouth Diallo, Pascal Handschumacher, Ousmane Faye, Amadou A. Sall Southeastern Senegal has become a major trade Hantaviruses cause hemorrhagic fever in humans worldwide. However, few hantavirus surveillance cam- area because of urbanization and substantial im- paigns occur in Africa. We detected Seoul orthohanta- provement of its road and rail networks in the late virus in black rats in Senegal, although we did not find 1990s (7). Within a few years, these changes led to the serologic evidence of this disease in humans. These rapid spread of a major invasive rodent species, the findings highlight the need for increased surveillance of black rat (Rattus rattus [family Murinae]), which is a hantaviruses in this region. reservoir for Seoul orthohantavirus (SEOV) (4,5,7). To assess the prevalence of hantaviruses in rodents, we antaviruses (family Hantaviridae, genus Ortho- screened for hantaviruses in R. rattus rats and com- Hhantavirus) are RNA viruses transmitted by aero- mensal or peridomestic co-existing rodents in 2012– solized excreta from infected rodents and shrews. In 2013, approximately 15 years after the 1998 opening humans, they cause hemorrhagic fever with renal of a tarred road in eastern Senegal. syndrome (more often observed in Asia and Europe) and cardiopulmonary syndrome (more common in The Study the Americas) (1). Only 1 case has been confirmed The national ethics committee for research of Senegal in Africa, in the Central African Republic in 1987 (2). -

Countries with Risk of Yellow Fever Transmission
ANNEX 1 Countries1 with risk of yellow fever transmission2 and countries requiring yellow fever vaccination Countries Countrieswithrisk Countriesrequiring Countriesrequiring ofyellowfever yellowfever yellowfever transmission vaccination vaccinationfor fortravellersarrivingtravellersfromall fromcountries countries withriskofyellow fevertransmission Afghanistan Yes Albania Yes Algeria Yes3 Angola Yes Yes Anguilla Yes3 AntiguaandBarbuda Yes Argentina Yes Australia Yes3 Bahamas Yes3 Bahrain Yes3 Bangladesh Yes Barbados Yes3 Belize Yes Benin Yes Yes Bhutan Yes3 Bolivia,Plurinational Stateof Yes Yes3 Botswana Yes3 Brazil Yes BruneiDarussalam Yes3 BurkinaFaso Yes Yes Burundi Yes Yes CaboVerde Yes3 Cambodia Yes3 Cameroon Yes Yes CentralAfrican Republic Yes Yes Chad Yes Yes China Yes3 ChristmasIsland Yes3 Colombia Yes Congo Yes Yes CostaRica Yes3 Côted’Ivoire Yes Yes DemocraticPeople’s RepublicofKorea Yes DemocraticRepublic oftheCongo Yes Yes Djibouti Yes Dominica Yes3 Ecuador Yes Yes Egypt Yes3 ElSalvador Yes3 EquatorialGuinea Yes Yes3 Eritrea Yes Ethiopia Yes Yes3 Fiji Yes3 FrenchGuiana Yes Yes3 FrenchPolynesia Yes3 Gabon Yes Yes Gambia Yes Yes3 Ghana Yes Yes3 Grenada Yes3 Guadeloupe Yes3 Guatemala Yes Guinea Yes Yes Guinea-Bissau Yes Yes Guyana Yes Yes Honduras Yes3 India Yes Indonesia Yes Iran(IslamicRepublic of) Yes Iraq Yes Jamaica Yes3 Jordan Yes Kazakhstan Yes Kenya Yes Yes Kiribati Yes Kyrgyzstan Yes3 LaoPeople’s DemocraticRepublic Yes Lebanon Yes3 Lesotho Yes3 Liberia