Carnivorous Plants List Jan Flísek
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quite a Few Reasons for Calling Carnivores 'The Most Wonderful
Annals of Botany 109: 47–64, 2012 doi:10.1093/aob/mcr249, available online at www.aob.oxfordjournals.org REVIEW Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’ Elz˙bieta Kro´l1,*,†, Bartosz J. Płachno2,†, Lubomı´r Adamec3, Maria Stolarz1, Halina Dziubin´ska1 and Kazimierz Tre˛bacz1 1Department of Biophysics, Institute of Biology, Maria Curie-Skłodowska University, Akademicka 19, 20-033 Lublin, Poland, 2Department of Plant Cytology and Embryology, Jagiellonian University, Grodzka 52, 31-044 Cracow, Poland and 3Institute of Botany AS CR, Dukelska´ 135, 37982 Trˇebonˇ, Czech Republic †These authors contributed equally to this work. * For correspondence. E-mail [email protected] Received: 30 May 2011 Returned for revision: 28 June 2011 Accepted: 8 August 2011 Published electronically: 21 September 2011 Downloaded from † Background A plant is considered carnivorous if it receives any noticeable benefit from catching small animals. The morphological and physiological adaptations to carnivorous existence is most complex in plants, thanks to which carnivorous plants have been cited by Darwin as ‘the most wonderful plants in the world’. When considering the range of these adaptations, one realizes that the carnivory is a result of a multitude of different features. † Scope This review discusses a selection of relevant articles, culled from a wide array of research topics on plant carnivory, and focuses in particular on physiological processes associated with active trapping and digestion of http://aob.oxfordjournals.org/ prey. Carnivory offers the plants special advantages in habitats where nutrient supply is scarce. Counterbalancing costs are the investments in synthesis and the maintenance of trapping organs and hydrolysing enzymes. -

Evolution of Unusual Morphologies in Lentibulariaceae (Bladderworts and Allies) And
Annals of Botany 117: 811–832, 2016 doi:10.1093/aob/mcv172, available online at www.aob.oxfordjournals.org REVIEW: PART OF A SPECIAL ISSUE ON DEVELOPMENTAL ROBUSTNESS AND SPECIES DIVERSITY Evolution of unusual morphologies in Lentibulariaceae (bladderworts and allies) and Podostemaceae (river-weeds): a pictorial report at the interface of developmental biology and morphological diversification Rolf Rutishauser* Institute of Systematic Botany, University of Zurich, Zurich, Switzerland * For correspondence. E-mail [email protected] Received: 30 July 2015 Returned for revision: 19 August 2015 Accepted: 25 September 2015 Published electronically: 20 November 2015 Background Various groups of flowering plants reveal profound (‘saltational’) changes of their bauplans (archi- tectural rules) as compared with related taxa. These plants are known as morphological misfits that appear as rather Downloaded from large morphological deviations from the norm. Some of them emerged as morphological key innovations (perhaps ‘hopeful monsters’) that gave rise to new evolutionary lines of organisms, based on (major) genetic changes. Scope This pictorial report places emphasis on released bauplans as typical for bladderworts (Utricularia,approx. 230 secies, Lentibulariaceae) and river-weeds (Podostemaceae, three subfamilies, approx. 54 genera, approx. 310 species). Bladderworts (Utricularia) are carnivorous, possessing sucking traps. They live as submerged aquatics (except for their flowers), as humid terrestrials or as epiphytes. Most Podostemaceae are restricted to rocks in tropi- http://aob.oxfordjournals.org/ cal river-rapids and waterfalls. They survive as submerged haptophytes in these extreme habitats during the rainy season, emerging with their flowers afterwards. The recent scientific progress in developmental biology and evolu- tionary history of both Lentibulariaceae and Podostemaceae is summarized. -

Carnivorous Plant Responses to Resource Availability
Carnivorous plant responses to resource availability: environmental interactions, morphology and biochemistry Christopher R. Hatcher A doctoral thesis submitted in partial fulfilment of requirements for the award of Doctor of Philosophy of Loughborough University November 2019 © by Christopher R. Hatcher (2019) Abstract Understanding how organisms respond to resources available in the environment is a fundamental goal of ecology. Resource availability controls ecological processes at all levels of organisation, from molecular characteristics of individuals to community and biosphere. Climate change and other anthropogenically driven factors are altering environmental resource availability, and likely affects ecology at all levels of organisation. It is critical, therefore, to understand the ecological impact of environmental variation at a range of spatial and temporal scales. Consequently, I bring physiological, ecological, biochemical and evolutionary research together to determine how plants respond to resource availability. In this thesis I have measured the effects of resource availability on phenotypic plasticity, intraspecific trait variation and metabolic responses of carnivorous sundew plants. Carnivorous plants are interesting model systems for a range of evolutionary and ecological questions because of their specific adaptations to attaining nutrients. They can, therefore, provide interesting perspectives on existing questions, in this case trait-environment interactions, plant strategies and plant responses to predicted future environmental scenarios. In a manipulative experiment, I measured the phenotypic plasticity of naturally shaded Drosera rotundifolia in response to disturbance mediated changes in light availability over successive growing seasons. Following selective disturbance, D. rotundifolia became more carnivorous by increasing the number of trichomes and trichome density. These plants derived more N from prey and flowered earlier. -

Florida Council of Bromeliad Societies, Inc
Florida Council of Bromeliad Societies, Inc. In This Issue: 2007 Shows and Sales Cold Hardy Bromeliads List Vol. 27 Issue 1 February 2007 FCBS Affiliated Societies and Representatives B. Guild Tampa Bay Caloosahatchee Tom Wolfe Vicky Chirnside 5211 Lake LeClare Road 951 Southland Road Lutz 33558 Venice 34293 813-961-1475 941-493-5825 [email protected] [email protected] Bob Teems Tom Foley 813-855-0938 239-458-4656 Broward County Fl. East Coast Jose Donayre Calandra Thurrott 1240 Jefferson St. 713 Breckenridge Drive Hollywood 33019-1807 Port Orange 32127 954-925-5112 386-761-4804 Jcadonayre @bellsouth.net [email protected] Colleen Hendrix Carolyn Schoenau 954-530-0076 352-372-6589 Central Florida F. West Coast Betsy McCrory Linda Sheetz 3615 Boggy Creek Rd. 1153 Williams Dr. S Kissimmee 34744 St. Petersburg 33705 407-348-2139 727-864-3165 [email protected] [email protected] Butch Force Brian Corey 407-886-4814 727-864-3165 South Florida Gainesville Juan Espinosa-Almodovar Al Muzzell P.O. Box 430722 P.O. Box 14442 Miami 33243 Gainesville 32604 305-667-6155 352-372-4576 [email protected] John R. Moxley Michael Michalski 352-528-0783 305-279-2416 (Continued on the inside back cover.) 2007 Bromeliad Extravaganza Presented by Florida Council of Bromeliad Societies Hosted by the Bromeliad Society of Broward County Saturday, September 29, 2007 at the Hilton Ft. Lauderdale Airport Hotel 1870 Griffin Rd. Dania Beach, FL 33004 954-920-3300 954-920-3348 (fax) Room rates: Single or double $89.00 Rates in effect until September 14, 2007 Sale, Banquet, Raffle and Rare Plant Auction will take place at the same location. -
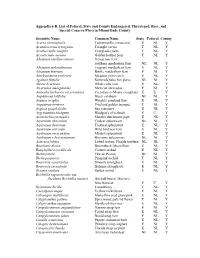
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

Checklist Das Spermatophyta Do Estado De São Paulo, Brasil
Biota Neotrop., vol. 11(Supl.1) Checklist das Spermatophyta do Estado de São Paulo, Brasil Maria das Graças Lapa Wanderley1,10, George John Shepherd2, Suzana Ehlin Martins1, Tiago Egger Moellwald Duque Estrada3, Rebeca Politano Romanini1, Ingrid Koch4, José Rubens Pirani5, Therezinha Sant’Anna Melhem1, Ana Maria Giulietti Harley6, Luiza Sumiko Kinoshita2, Mara Angelina Galvão Magenta7, Hilda Maria Longhi Wagner8, Fábio de Barros9, Lúcia Garcez Lohmann5, Maria do Carmo Estanislau do Amaral2, Inês Cordeiro1, Sonia Aragaki1, Rosângela Simão Bianchini1 & Gerleni Lopes Esteves1 1Núcleo de Pesquisa Herbário do Estado, Instituto de Botânica, CP 68041, CEP 04045-972, São Paulo, SP, Brasil 2Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas – UNICAMP, CP 6109, CEP 13083-970, Campinas, SP, Brasil 3Programa Biota/FAPESP, Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas – UNICAMP, CP 6109, CEP 13083-970, Campinas, SP, Brasil 4Universidade Federal de São Carlos – UFSCar, Rod. João Leme dos Santos, Km 110, SP-264, Itinga, CEP 18052-780, Sorocaba, SP, Brasil 5Departamento de Botânica – IBUSP, Universidade de São Paulo – USP, Rua do Matão, 277, CEP 05508-090, Cidade Universitária, Butantã, São Paulo, SP, Brasil 6Departamento de Ciências Biológicas, Universidade Estadual de Feira de Santana – UEFS, Av. Transnordestina, s/n, Novo Horizonte, CEP 44036-900, Feira de Santana, BA, Brasil 7Universidade Santa Cecília – UNISANTA, R. Dr. Oswaldo Cruz, 266, Boqueirão, CEP 11045-907, -

FINAL REPORT PSRA Vegetation Monitoring 2005-2006 PC P502173
Rare Plants and Their Locations at Picayune Strand Restoration Area: Task 4a FINAL REPORT PSRA Vegetation Monitoring 2005-2006 PC P502173 Steven W. Woodmansee and Michael J. Barry [email protected] December 20, 2006 Submitted by The Institute for Regional Conservation 22601 S.W. 152 Avenue, Miami, Florida 33170 George D. Gann, Executive Director Submitted to Mike Duever, Ph.D. Senior Environmental Scientist South Florida Water Management District Fort Myers Service Center 2301 McGregor Blvd. Fort Myers, Florida 33901 Table of Contents Introduction 03 Methods 03 Results and Discussion 05 Acknowledgements 38 Citations 39 Tables: Table 1: Rare plants recorded in the vicinity of the Vegetation Monitoring Transects 05 Table 2: The Vascular Plants of Picayune Strand State Forest 24 Figures: Figure 1: Picayune Strand Restoration Area 04 Figure 2: PSRA Rare Plants: Florida Panther NWR East 13 Figure 3: PSRA Rare Plants: Florida Panther NWR West 14 Figure 4: PSRA Rare Plants: PSSF Northeast 15 Figure 5: PSRA Rare Plants: PSSF Northwest 16 Figure 6: PSRA Rare Plants: FSPSP West 17 Figure 7: PSRA Rare Plants: PSSF Southeast 18 Figure 8: PSRA Rare Plants: PSSF Southwest 19 Figure 9: PSRA Rare Plants: FSPSP East 20 Figure 10: PSRA Rare Plants: TTINWR 21 Cover Photo: Bulbous adder’s tongue (Ophioglossum crotalophoroides), a species newly recorded for Collier County, and ranked as Critically Imperiled in South Florida by The Institute for Regional Conservation taken by the primary author. 2 Introduction The South Florida Water Management District (SFWMD) plans on restoring the hydrology at Picayune Strand Restoration Area (PSRA) see Figure 1. -

Natural Forest Community Delineation Methods
Natural Forest Community Delineation Methods Keith A. Bradley and George D. Gann February 2, 2005 The Institute for Regional Conservation 22601 S.W. 152 Avenue; Miami, Florida 33170 George D. Gann, Executive Director Introduction The Natural Forest Community (NFC) system was established in 1984 under ordinance 89-9, Chapter 24-60 of the Miami-Dade County Code. The ordinance provides legal protection for sites designated by the county as NFCs: “Natural Forest Community shall mean all stands of trees (including their associated understory) which were designated as Natural Forest Communities on the Dade County Natural Forest Community Maps and approved by the Board of County Commissioners, pursuant to Resolution No. R-1764-84.” Factors for reviewing proposed Natural Forest Community sites in the original ordinance included: 1) Presence of endangered, threatened, rare, or endemic species (plants or animals); 2) Plant species diversity on the site; 3) Size of trees; 4) Size of site; 5) Wildlife habitat value; 6) Geological features; and 7) Percentage of site covered by non-native plant species. As required by the ordinance, quantitative evaluation criteria were developed by DERM incorporating the above factors, including separate criteria for the delineation of hardwood hammocks and pinelands. These criteria have become outdated as more scientifically rigorous criteria for delineating natural areas have been developed since 1984, primarily relating to delineation of wetland habitats. Some of the methods used to delineate NFCs by DERM proved to be ineffective including the establishment of transects to measure plant species cover and diversity on each site. As specified in section 151 of the ordinance, evaluation criteria may be revised occasionally. -
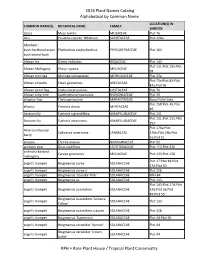
2016 Plant Names Catalog Alphabetical by Common Name
2016 Plant Names Catalog Alphabetical by Common Name LOCATION(S) IN COMMON NAME(S) BOTANICAL NAME FAMILY GARDEN abaca Musa textilis MUSACEAE Plot 76 abiu Pouteria caimito 'Whitman' SAPOTACEAE Plot 128a Abraham- bush:hardhead:scipio- Phyllanthus epiphyllanthus PHYLLANTHACEAE Plot 164 bush:sword-bush African iris Dietes iridioides IRIDACEAE Plot 143 Plot 131:Plot 19a:Plot African Mahogany Khaya nyasica MELIACEAE 58 African moringa Moringa stenopetala MORINGACEAE Plot 32a Plot 71a:Plot 83:Plot African oil palm Elaeis guineensis ARECACEAE 84a:Plot 96 African spiral flag Costus lucanusianus COSTACEAE Plot 76 African tulip-tree Spathodea campanulata BIGNONIACEAE Plot 29 alligator flag Thalia geniculata MARANTACEAE Royal Palm Lake Plot 158:Plot 45:Plot allspice Pimenta dioica MYRTACEAE 46 Amazon lily Eucharis x grandiflora AMARYLLIDACEAE Plot 131 Plot 131:Plot 151:Plot Amazon-lily Eucharis amazonica AMARYLLIDACEAE 152 Plot 176a:Plot American beauty Callicarpa americana LAMIACEAE 176b:Plot 19b:Plot berry 3a:Plot 51 anaqua Ehretia anacua BORAGINACEAE Plot 52 anchovy pear Grias cauliflora LECYTHIDACEAE Plot 112:Plot 32b andiroba:bastard Carapa guianensis MELIACEAE Plot 133:Plot 158 mahogany Plot 17:Plot 18:Plot angel's trumpet Brugmansia aurea SOLANACEAE 27d:Plot 50 angel's trumpet Brugmansia aurea x SOLANACEAE Plot 32b angel's trumpet Brugmansia 'Ecuador Pink' SOLANACEAE RPH-B4 angel's trumpet Brugmansia sp. SOLANACEAE Plot 133 Plot 143:Plot 27d:Plot angel's trumpet Brugmansia suaveolens SOLANACEAE 32b:Plot 3a:Plot 49:Plot 50 Brugmansia suaveolens -

Why Are Plants Carnivorous?
1 ECOPHYSIOLOGICAL LOOK AT PLANT CARNIVORY: Why are plants carnivorous? LUBOMÍR ADAMEC Institute of Botany, Section of Plant Ecology Dukelská 135, CZ-379 82 T řebo ň, Czech Republic 1. Introduction About 650 species of vascular carnivorous (Latin: carnis – flesh, vorare – to swallow) plants occur throughout the world (e.g., Rice, 2006) out of the total of about 300,000 species of vascular plants. Carnivorous plants belong to 15-18 genera of 8-9 botanical families and 5 orders (Givnish, 1989; Juniper et al., 1989; Müller et al., 2004; Heubl et al., 2006; Porembski and Barthlott, 2006; Studni čka, 2006). Due to many remarkable and striking morphological, anatomical, physiological, and ecological features, carnivorous plants have always attracted considerable interest of both researchers and gardeners. Nevertheless, the degree and extent of knowledge of the main disciplines studying this particular ecological functional plant group has always considerably lagged behind the study of non-carnivorous plants. However, similar to the dynamically growing knowledge of non-carnivorous plants, the study of carnivorous plants has developed very rapidly and progressively within the last decade, mainly due to the use of modern molecular taxonomic approaches. Also, modern ecophysiological research of carnivorous plants has progressed considerably within the last decade and has elucidated most of the particulars of carnivorous plants. Thus we are increasingly more able to discuss to what extent carnivorous plants are unique from or common with “normal” non-carnivorous plants. The aim of this paper is to classify and review recent experimental results and concepts concerning plant carnivory from an ecophysiological point of view, with an emphasis on mineral nutrition, growth characteristics, and comparison of aquatic and terrestrial carnivorous plants. -

The Roots of Carnivorous Plants
Plant and Soil (2005) 274:127–140 Ó Springer 2005 DOI 10.007/s11104-004-2754-2 The roots of carnivorous plants Wolfram Adlassnig1, Marianne Peroutka1, Hans Lambers2 & Irene K. Lichtscheidl1,3 1Institute of Ecology and Conservation Biology, University of Vienna, Althanstrasse 14, 1090 Vienna, Austria. 2School of Plant Biology, Faculty of Natural and Agricultural Sciences, The University of Western Australia, Crawley WA 6009, Australia. 3Corresponding author* Received 30 April 2004. Accepted in revised form 31 August 2004 Key words: carnivorous plants, insectivorous plants, morphology, nutrition, root Abstract Carnivorous plants may benefit from animal-derived nutrients to supplement minerals from the soil. Therefore, the role and importance of their roots is a matter of debate. Aquatic carnivorous species lack roots completely, and many hygrophytic and epiphytic carnivorous species only have a weakly devel- oped root system. In xerophytes, however, large, extended and/or deep-reaching roots and sub-soil shoots develop. Roots develop also in carnivorous plants in other habitats that are hostile, due to flood- ing, salinity or heavy metal occurance. Information about the structure and functioning of roots of car- nivorous plants is limited, but this knowledge is essential for a sound understanding of the plants’ physiology and ecology. Here we compile and summarise available information on: (1) The morphology of the roots. (2) The root functions that are taken over by stems and leaves in species without roots or with poorly developed root systems; anchoring and storage occur by specialized chlorophyll-less stems; water and nutrients are taken up by the trap leaves. (3) The contribution of the roots to the nutrient supply of the plants; this varies considerably amongst the few investigated species. -

CPN V37n3 September 2008
ON GROWING UTRICULARIA SECT. ORCHIDIOIDES TRAVIS H. WYMAN • 4063 Indian Lakes Circle • Stone Mountain GA 30083 • USA • [email protected] Keywords: cultivation: Utricularia, Orchidioides. Introduction The species in the genus Utricularia are grouped into many sections. The species in the sec- tion Orchidioides are very popular and sought after by growers because of their large orchid-like flowers, their (usually) large leaves that dwarf those of most other species, and their interesting habit of producing tubers. This section includes the species: U. alpina, U. asplundii, U. jameso- niana, U. endresii, U. praetermissa, U. quelchii, U. campbelliana, U. unifolia, and U. buntin- giana. However, section Orchidioides species often carry such a stigma of being so difficult to grow and requiring such complicated techniques or challenging conditions that, despite the inter- est in the group, only U. alpina is commonly found in collections. In truth, anyone who is able to successfully grow highland Nepenthes or Heliamphora should be able to grow a number of the species in this section with little difficulty. In this article I will detail how I have grown and cur- rently grow the species from this section in the hopes that my experiences will help to demysti- fy these plants. Growers and carnivorous plant articles often clump the species in section Orchidioides togeth- er with those in section Iperua, but they have different cultivation requirements. I currently grow various clones of all the section Orc hidioides e xcept the final two species listed above. I also grow the hybrids U. quelchii × praetermissa ‘Jitka’, U. alpina × endresii and U.