Genesis and Influencing Factors of the Colour of Chrysoprase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Syntectonic Mobility of Supergene Nickel Ores of New Caledonia (Southwest Pacific)
Syntectonic mobility of supergene nickel ores of New Caledonia (Southwest Pacific). Evidence from faulted regolith and garnierite veins. Dominique Cluzel, Benoit Vigier To cite this version: Dominique Cluzel, Benoit Vigier. Syntectonic mobility of supergene nickel ores of New Caledonia (Southwest Pacific). Evidence from faulted regolith and garnierite veins.. Resource Geology, Wiley- Blackwell publishing, 2008, 58 (2), pp.161 - 170. 10.1111/j.1751-3928.2008.00053.x. hal-00161201 HAL Id: hal-00161201 https://hal.archives-ouvertes.fr/hal-00161201 Submitted on 10 Jul 2007 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Syntectonic mobility of supergene nickel ores of New Caledonia (Southwest Pacific). Evidence from faulted regolith and garnierite veins. Dominique CLUZEL and Benoit VIGIER Institut des Sciences de la Terre d'Orléans, ISTO, UMR 6113, University of Orleans, BP 6759, 45067 Orléans Cedex 2, France. [email protected] Running title: Syntectonic mobility of supergene nickel ores Abstract Supergene nickel deposits of New Caledonia that have been formed in the Neogene by weathering of obducted ultramafic rocks are tightly controlled by fracture development. The relationship of tropical weathering and tectonic structures, faults and tension gashes, have been investigated in order to determine whether fractures have play a passive role only, as previously thought; or alternatively, if brittle tectonics was acting together with alteration. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Download the Scanned
American Mineralogist, Volume 59, pages 906-918, 1974 Domainsin Minerals Rosnnr E. NpwNnarvr Materials ResearchLaboratory, The PennsylaaniaState Uniuersity, Uniuersity Park, Pennsyluania16802 Abstract Mimetic twinning in minerals is reviewed in terms of the tensor properties of the orientation states, showing which forces are eftective in moving domain walls. Following the Aizu method, various types of ferroic species are developed frorn the free energy function. Examples of ferroelectric, ferromagnetic, ferroelastic, ferrobielectric, ferrobimagnetic, ferrobielastic, ferro- elastoelectric, ferromagnetoelastic, and ferromagnetoelectric minerals are described. Introduction and ferromagnetoelectric.As explained later, each Twinning is widely used in mineral identification type of domain reorientationarises from a particular and in elucidating the formation conditions of rocks. term in the free energy function. The distribution of transformation twins in rock- A ferroic crystal contains two or more possible forming minerals enables one to establish the orientationstates or domains;under a suitably chosen thermal processes that have occurred in the rock. driving force the domain walls move, switching the Mechanical twinning is studied by petrologists in crystal from one orientation stateto another. Switch- the analysis of flow effects. In rock magnetism, it ing may be accomplishedby mechanicalstress (a), is the arrangement of ferromagnetic domains which electricfield (E), magneticfield (I/), or somecombina- determines remanent magnetization. These are but tion of the three. Ferroelectric, ferroelastic, and a few examples of twin phenomena in minerals. ferromagneticmaterials are well known examplesof In the past twinned crystals have been classified primary ferroic crystals in which the orientation according to twin-laws and morphology, or accord- statesdiffer respectivelyin spontaneouspolarization ing to their mode of origin, or on a structural basis, P,",, spontaneousstrain 6r"r and spontaneous but there is another classification scheme which magnetizatiorrMr"t. -

Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite
minerals Article Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite Arun Kumar 1,2 , Michele Cassetta 1, Marco Giarola 3, Marco Zanatta 4 , Monique Le Guen 5, Gian Domenico Soraru 6 and Gino Mariotto 1,* 1 Department of Computer Science, University of Verona, 37134 Verona, Italy; [email protected] (A.K.); [email protected] (M.C.) 2 CNR-Institute for Microelectronics and Microsystems, Agrate Brianza, 20864 Agrate, Italy 3 Centro Piattaforme Tecnologiche (CPT), University of Verona, 37134 Verona, Italy; [email protected] 4 Department of Physics, University of Trento, 38123 Povo, Italy; [email protected] 5 Innovation Technology Direction, ERAMET IDEAS, 78190 Trappes, France; [email protected] 6 Department of Industrial Engineering, University of Trento, 38123 Povo, Italy; [email protected] * Correspondence: [email protected] Abstract: This study is focused on the vibrational and microstructural aspects of the thermally induced transformation of serpentine-like garnierite into quartz, forsterite, and enstatite occur- ring at about 620 ◦C. Powder specimens of garnierite were annealed in static air between room temperature and 1000 ◦C. The kinetic of the transformation was investigated by means of thermo- gravimetric and differential thermal analysis, and the final product was extensively characterized via micro-Raman spectroscopy and X-ray diffraction. Our study shows that serpentine-like garnierite consists of a mixture of different mineral species. Furthermore, these garnierites and their compo- sition can provide details based on the mineralogy and the crystalline phases resulting from the thermal treatment. Citation: Kumar, A.; Cassetta, M.; Giarola, M.; Zanatta, M.; Le Guen, M.; Keywords: garnierite; phase transformation; TGA/DSC; XRD; micro-Raman spectroscopy Soraru, G.D.; Mariotto, G. -

Thermal and Infrared Studies of Garnierite from the Soroako Nickeliferous Laterite Deposit, Sulawesi, Indonesia
Indonesian Journal of Geology, Vol. 7 No. 2 June 2012: 77-85 Thermal and Infrared Studies of Garnierite from the Soroako Nickeliferous Laterite Deposit, Sulawesi, Indonesia Analisis Termal dan Inframerah Garnierit dari Endapan Laterit Nikel Saroako, Sulawesi, Indonesia SUFRIADIN1,3*, A. IDRUS1, S. PRAMUMIJOYO1, I W. WARMADA1, I. NUR1,3, A. IMAI2, A.M. IMRAN3, and KAHARUDDIN3 1Department of Geological Engineering, Gadjah Mada University, Yogyakarta 55281, Indonesia 2Department of Earth Science and Technology, Akita University, Akita 010-8512, Japan 3Department of Geological Engineering, Hasanuddin University, Makassar 90245, Indonesia ABSTRACT Mineralogical characterization of some garnierite samples from Soroako have been conducted using X-ray diffraction, thermal analysis, and infrared spectroscopy methods. XRD patterns reveal the samples mainly containing the mixture of kerolite (talc-like phase) and serpentine with minor smectite, sepiolite, and silica. Thermal analyses of garnierite samples indicated by DTA curves are in good agreement with patterns that have been reported in literature. Three endothermic peaks normally occur in the ranges between 58º C and <800º C illustrating three steps of weight losses: adsorbed, bound, and hydroxyl/crystal water. One additional weight loss in low temperature region of sepiolite is corresponding to the lost of zeolitic water. Infrared spectra appeared in 3800 - 3200 cm-1 region generally exhibit broad absorption bands, indicating low crystallinities of studied samples and can be assigned to the presence of hydroxyl group bonded to octahedral coordina- tion mainly Mg atom. The bands observed at 1660 cm-1, 1639 cm-1, 1637 cm-1, and 1633 cm-1 in all samples indicate water molecules. FTIR spectra displaying the strong bands at 1045 cm-1, 1038 cm-1, and 1036 cm-1 could be related to the presence of Si-O-Si bonds linking to tetrahedral coordination. -

DOGAMI MP-20, Investigations of Nickel in Oregon
0 C\1 a: w a.. <( a.. en ::::> 0 w z <( __j __j w () en � INVESTIGATIONS OF NICKEL IN OREGON STATE OF OREGON DEPARTMENT OF GEOL.OGY AND MINERAL. IN OUSTRIES DONAL.D .A HUL.L. STATE GEOLOGIST 1978 STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL INDUSTRIES 1069 State Office Building, Portland, Oregon 97201 MISCELLANEOUS PAPER 20 INVESTIGATIONS OF NICKEL IN OREGON Len Ramp, Resident Geologist Grants Pass Field Office Oregon Department of Geology and Mineral Industries Conducted in conformance with ORS 516.030 . •. 5 1978 GOVERNING BOARD Leeanne MacColl, Chairperson, Portland Talent Robert W. Doty STATE GEOLOGIST John Schwabe Portland Donald A. Hull CONTENTS INTRODUCTION -- - ---- -- -- --- Purpose and Scope of this Report Acknowledgments U.S. Nickel Industry GEOLOGY OF LATERITE DEPOSITS - -- - 3 Previous Work - - - - --- 3 Ultramafic Rocks - ----- --- 3 Composition - - -------- - 3 Distribution ------ - - - 3 Structure - 3 Geochemistry of Nickel ---- 4 Chemical Weathering of Peridotite - - 4 The soi I profile ------- 5 M i nero I ogy -- - ----- 5 Prospecting Guides and Techniques- - 6 OTHER TYPES OF NICKEL DEPOSITS - - 7 Nickel Sulfide Deposits- - - - - - 7 Deposits in Oregon 7 Other areas --- 8 Prospecting techniques 8 Silica-Carbonate Deposits - -- 8 DISTRIBUTION OF LATERITE DEPOSITS - ------ 9 Nickel Mountain Deposits - - ------ --------- 9 Location --------------- --- 9 Geology - ------- ----- 11 Ore deposits ----------- - -- 11 Soil mineralogy - ------- 12 Structure --- ---- ---- 13 Mining and metallurgy ------------ ---- 13 Production- -

Nickel Minerals from Barberton, South Africa: I
American Mineralogist, Volume 58, pages 733-735, 1973 NickelMinerals from Barberton,South Africa: Vl. Liebenbergite,A NickelOlivine SvsneNoA. nn Wnar Nationnl Institute lor Metallurgy, I Yale Road.,Milner Park, I ohannesburg,South Alrica Lnwrs C. ClI.x Il. S. GeologicalSuruey, 345 Middlefield Road, Menlo Park, California 94025 Abstract Liebenbergite, a nickel olivine from the mineral assemblage trevorite-liebenbergite-nickel serpentine-nickel ludwigite-bunsenite-violarite-millerite-gaspeite-nimite, is described mineral- ogically.Ithasa-1.820,P-1.854,7-1.888,ZVa-88",specificgravity-4'60,Mohs hardness- 6 to 6.5, a - 4.727, b - 10.191,c = 5.955A, and Z - 4. X-ray powder data (48 lines) were indexed according to the space grottp Pbnm. The mean chemical composi- tion, calculated from electron microprobe analyses of eight separate liebenbergite grains, gives the mineral formula: (NL eMgoaCoo,sFeo o)Sio "nOn The name is for W. R. Liebenberg, Deputy Director-General of the National Institute for Metallurgy, South Africa. Introduction mission on New Minerals and Mineral Names (rMA). A re-investigationof the trevorite deposit at Bon Accord in the Barberton Mountain Land, South Experimental Methods Africa, led to the discovery of two peculiar but distinct nickel mineral assemblages.Minerals from The refractive indices were determined by the the assemblagewillemseite-nimite-feroan trevorite- conventional liquid immersion method using a reevesite-millerite-violarite-goethitehave been de- sodium lamp as light source.The optical and crystal scribed in earlier papers in this series (de Waal, morphologicalparameters were studiedwith the aid 1969, l97oa, 1970b;de Waal and Viljoen, I97L). of a universal stage. -
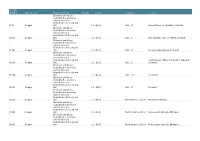
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

Swelling Capacity of Mixed Talc-Like/Stevensite Layers in White/Green Clay
This is a preprint, the final version is subject to change, of the American Mineralogist (MSA) Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2020-6984 1 1 Plagcheck: no concerns 2 Tables?: 3 small 3 Word Count: ~9,100 4 Prod notes: make sure tables in file before RE 5 6 7 8 Swelling capacity of mixed talc-like/stevensite layers in white/green clay 9 infillings (‘deweylite’/‘garnierite’) from serpentine veins of faulted 10 peridotites, New Caledonia 11 REVISION 2 12 Lionel FONTENEAU 1, Laurent CANER 2*, Sabine PETIT 2, Farid JUILLOT 3, Florian 13 PLOQUIN 3, Emmanuel FRITSCH 3 14 15 1Corescan Pty Ltd, 1/127 Grandstand Road, 6104 Ascot, WA, Australia 16 2 Université de Poitiers, Institut de Chimie des Milieux et Matériaux de Poitiers, IC2MP UMR 17 7285 CNRS, 5 rue Albert Turpain, TSA51106, 86073 Poitiers cedex 9, France 18 * Corresponding author, e-mail: [email protected] 19 3 Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie (IMPMC), Sorbonne 20 Universités – Université Pierre et Marie Curie UPMC, UMR CNRS 7590, Museum National 21 d’Histoire Naturelle, UMR IRD 206, 101 Promenade Roger Laroque, Anse Vata, 98848, 22 Nouméa, New Caledonia 23 24 25 Abstract: White (Mg-rich) and green (Ni-rich) clay infillings (‘deweylite’/‘garnierite’) found 26 in serpentine veins of faulted peridotite formations from New Caledonia consist of an intimate 27 mixture of fine-grained and poorly ordered 1:1 and 2:1 layer silicates, commonly referred to 28 as non-expandable serpentine-like (SL) and talc-like (TL) minerals. -

Gem-Quality Chrysoprase from Haneti-Itiso Area, Central Tanzania
GEM-QUALITY CHRYSOPRASE FROM HANETI-ITISO AREA, CENTRAL TANZANIA KARI A. KINNUNEN and ELIAS J. MALISA KINNUNEN, KARI A. and MALISA ELIAS J., 1990: Gem-quality Chrysoprase from Haneti-Itiso area, Central Tanzania. Bull. Geol. Soc. Finland 62, Part 2, 157—166. Gem-quality, apple-green, Ni-bearing chalcedonic quartz occurs as near-surface veins in silicified serpentinite in the Haneti-Itiso area, Central Tanzania. AAS de- terminations revealed a high Ni content, 0.55 wt.%, and low Co and Cr contents of 120 and 1 ppm respectively. NAA determination revealed near chondritic REE contents. X-ray diffraction determinations showed that the Chrysoprase consists main- ly of alpha quartz with some opal-CT. The gemmological properties are: refractive indices from 1.548 to 1.553 (±0.002), mean specific gravity 2.56, hardness about 7 on Moh's scale, inert to ultraviolet radiation, green through Chelsea filter, and absorption in the deep red and violet part of the optical absorption spectrum. The results confirm the identity of the material as Chrysoprase. Microscopically the Tanzanian Chrysoprase consists of spherules which are high- ly disordered, concentric, and composed of bipyramidal quartz, chalcedony, quart- zine, and opal-A. They were classified into four main types according to the shell arrangement. The diameter of the spherules ranged from 40 um to 77 um. Fluid inclusion types in the bipyramidal quartz were monophasic, low-temperature type. The spherules, silica types and REE contents suggest that this Chrysoprase was deposited by evaporation of surface waters connected with the silicification of the serpentinites. Genetically analogous formations, common in Africa, include M-fabric type, weathering profile silcretes. -

Characterisation of Ni Silicate-Bearing Minerals by UV-Vis-NIR Spectrscopy
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Bayyareddy, Jagannadha, Frost, Ray,& Dickfos, Marilla (2009) Characterisation of Ni silicate-bearing minerals by UV-vis-NIR spec- trscopy: Effect of Ni substitution in hydrous Ni-Mg silicates. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 71(5), pp. 1762-1768. This file was downloaded from: https://eprints.qut.edu.au/15661/ c Copyright 2009 Elsevier Reproduced in accordance with the copyright policy of the publisher. Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. https://doi.org/10.1016/j.saa.2008.06.030 QUT Digital Repository: http://eprints.qut.edu.au/ Reddy, B. Jagannadha and Frost, Ray L. and Dickfos, Marilla J. (2009) Characterisation of Ni silicate-bearing minerals by UV-Vis-NIR spectroscopy - Effect of Ni substitution in hydrous Ni-Mg silicates . Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 71:pp. 1762-1768. © Copyright 2009 Elsevier 1 2 Characterisation of Ni silicate-bearing minerals by UV-Vis-NIR spectroscopy 3 -Effect of Ni substitution in hydrous Ni-Mg silicates 4 5 B. Jagannadha Reddy, Ray L. Frost •and Marilla J. Dickfos 6 7 Inorganic Materials Research program, School of Physical and Chemical Sciences, 8 Queensland University of Technology, GPO Box 2434, Brisbane Queensland 4001, 9 Australia. -

The Phase Diversity of Nickel Phyllosilicates from New Caledonia: an Investigation Using Transmission Electron Microscopy (TEM) STUDENT: Brittany A
THE PHASE DIVERSITY OF NICKEL PHYLLOSILICATES FROM NEW CALEDONIA: AN INVESTIGATION USING TRANSMISSION ELECTRON MICROSCOPY (TEM) A THESIS SUBMITTED TO THE GRADUATE SCHOOL IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE MASTER OF SCIENCE BY BRITTANY CYMES DR. KIRSTEN NICHOLSON – ADVISOR BALL STATE UNIVERSITY MUNCIE, INDIANA MAY 2016 ABSTRACT THESIS: The Phase Diversity of Nickel Phyllosilicates from New Caledonia: An Investigation Using Transmission Electron Microscopy (TEM) STUDENT: Brittany A. Cymes DEGREE: Master of Science COLLEGE: Sciences and Humanities DATE: May, 2016 PAGES: 136 New Caledonia is a French territorial island in the Southwest Pacific with an economy heavily dependent upon nickel-mining, being the 5th largest producer worldwide. The nickel deposits result from tropical lateritic weathering of ophiolite units that were emplaced during the late Eocene. The ultramafic units weathered and continue to weather into bright green Ni-rich phyllosilicates colloquially referred to as „garnierite‟. Detailed investigations of „garnierite‟ using transmission electron microscopy (TEM) have been carried out in other locations to characterize important nanoscale features; however, none have been undertaken in New Caledonia. In this investigation, ten samples of „garnierite‟ from New Caledonia were examined with powder X-ray diffraction (XRD) and TEM with energy dispersive spectrometry (EDS). The XRD results show the phyllosilicate material to be comprised of highly disordered talc- and serpentine-like minerals with minor chlorite indicated by significantly broad reflections and two- i dimensional diffraction bands, consistent with published data. TEM analysis, however, revealed new information. Talc-like minerals occur as fluffy aggregates of crystals with lattices with approximately 10 Å d-spacing and significant crystallographic disorder and also as aggregates of larger crystals with more orderly lattices; these two varieties represent the kerolite-pimelite series and the talc- willemseite series, respectively.