The Picking Table Volume 8, No. 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sarkinite Mn (Aso4)(OH)
2+ Sarkinite Mn2 (AsO4)(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic. Point Group: 2/m. Crystals typically thick tabular {100}, elongated, to 4 mm, short prismatic, or tabular along [010]. May be crudely spherical, granular massive. Physical Properties: Cleavage: On {100}, distinct. Fracture: Subconchoidal to uneven. Hardness = 4–5 D(meas.) = 4.08–4.18 D(calc.) = 4.20 Optical Properties: Semitransparent. Color: Flesh-red to dark blood-red, rose-red, orange, orange-brown, brown, reddish yellow to yellow; pale rose to yellow in transmitted light. Streak: Rose-red to yellow. Luster: Greasy. Optical Class: Biaxial (–). Pleochroism: Weak. Orientation: Y = b; X ∧ c = –54◦. Dispersion: r< v. Absorption: X > Z > Y. α = 1.790–1.793 β = 1.794–1.807 γ = 1.798–1.809 2V(meas.) = 83◦ Cell Data: Space Group: P 21/a. a = 12.779(2) b = 13.596(2) c = 10.208(2) β = 108◦530 Z=16 X-ray Powder Pattern: Pajsberg [Harstigen mine, near Persberg], Sweden. 3.18 (10), 3.04 (10), 3.29 (9), 3.48 (8), 2.90 (7), 2.65 (6), 6.0 (3) Chemistry: (1) (2) (3) (1) (2) (3) P2O5 0.21 ZnO 0.15 As2O5 41.60 44.09 43.23 PbO 0.25 CO2 0.76 MgO 0.98 0.19 FeO 0.13 0.02 CaO 1.40 0.29 MnO 51.60 51.77 53.38 H2O 3.06 [3.40] 3.39 CuO 0.01 insol. 0.38 Total 100.37 [99.92] 100.00 (1) Pajsberg [Harstigen mine, near Persberg], Sweden. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite
minerals Article Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite Arun Kumar 1,2 , Michele Cassetta 1, Marco Giarola 3, Marco Zanatta 4 , Monique Le Guen 5, Gian Domenico Soraru 6 and Gino Mariotto 1,* 1 Department of Computer Science, University of Verona, 37134 Verona, Italy; [email protected] (A.K.); [email protected] (M.C.) 2 CNR-Institute for Microelectronics and Microsystems, Agrate Brianza, 20864 Agrate, Italy 3 Centro Piattaforme Tecnologiche (CPT), University of Verona, 37134 Verona, Italy; [email protected] 4 Department of Physics, University of Trento, 38123 Povo, Italy; [email protected] 5 Innovation Technology Direction, ERAMET IDEAS, 78190 Trappes, France; [email protected] 6 Department of Industrial Engineering, University of Trento, 38123 Povo, Italy; [email protected] * Correspondence: [email protected] Abstract: This study is focused on the vibrational and microstructural aspects of the thermally induced transformation of serpentine-like garnierite into quartz, forsterite, and enstatite occur- ring at about 620 ◦C. Powder specimens of garnierite were annealed in static air between room temperature and 1000 ◦C. The kinetic of the transformation was investigated by means of thermo- gravimetric and differential thermal analysis, and the final product was extensively characterized via micro-Raman spectroscopy and X-ray diffraction. Our study shows that serpentine-like garnierite consists of a mixture of different mineral species. Furthermore, these garnierites and their compo- sition can provide details based on the mineralogy and the crystalline phases resulting from the thermal treatment. Citation: Kumar, A.; Cassetta, M.; Giarola, M.; Zanatta, M.; Le Guen, M.; Keywords: garnierite; phase transformation; TGA/DSC; XRD; micro-Raman spectroscopy Soraru, G.D.; Mariotto, G. -

DOGAMI MP-20, Investigations of Nickel in Oregon
0 C\1 a: w a.. <( a.. en ::::> 0 w z <( __j __j w () en � INVESTIGATIONS OF NICKEL IN OREGON STATE OF OREGON DEPARTMENT OF GEOL.OGY AND MINERAL. IN OUSTRIES DONAL.D .A HUL.L. STATE GEOLOGIST 1978 STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL INDUSTRIES 1069 State Office Building, Portland, Oregon 97201 MISCELLANEOUS PAPER 20 INVESTIGATIONS OF NICKEL IN OREGON Len Ramp, Resident Geologist Grants Pass Field Office Oregon Department of Geology and Mineral Industries Conducted in conformance with ORS 516.030 . •. 5 1978 GOVERNING BOARD Leeanne MacColl, Chairperson, Portland Talent Robert W. Doty STATE GEOLOGIST John Schwabe Portland Donald A. Hull CONTENTS INTRODUCTION -- - ---- -- -- --- Purpose and Scope of this Report Acknowledgments U.S. Nickel Industry GEOLOGY OF LATERITE DEPOSITS - -- - 3 Previous Work - - - - --- 3 Ultramafic Rocks - ----- --- 3 Composition - - -------- - 3 Distribution ------ - - - 3 Structure - 3 Geochemistry of Nickel ---- 4 Chemical Weathering of Peridotite - - 4 The soi I profile ------- 5 M i nero I ogy -- - ----- 5 Prospecting Guides and Techniques- - 6 OTHER TYPES OF NICKEL DEPOSITS - - 7 Nickel Sulfide Deposits- - - - - - 7 Deposits in Oregon 7 Other areas --- 8 Prospecting techniques 8 Silica-Carbonate Deposits - -- 8 DISTRIBUTION OF LATERITE DEPOSITS - ------ 9 Nickel Mountain Deposits - - ------ --------- 9 Location --------------- --- 9 Geology - ------- ----- 11 Ore deposits ----------- - -- 11 Soil mineralogy - ------- 12 Structure --- ---- ---- 13 Mining and metallurgy ------------ ---- 13 Production- -
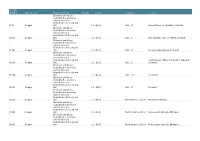
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

The Picking Table Volume 27, No. 1 – Spring 1986
TABLE JOURNAL of the FRANKLIN-OGDENSBURG MINERALOGICAL SOCIETY, INC. SPRING. 1986 VOLUME 27, NO.l The contents of The Picking Table are licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. F.QM.S. Notes prise a spectacular fluorescent display. For PRESIDENT'S MESSAGE years the Gerstmann Mineral Museum has displayed the collection for the delight and With the melting of the snow, the rocks of education of amateur and professional mineralo- the Buckwheat Dump emerge from their white gists alike. The Franklin Mineral Museum mantle, and the seismic tremors rumble through is most grateful to Arthur and Harriet Mitteldorf the souls of the collector community. Whatever for this most generous donation and to Ewald Spring may mean to the average mortal, to Gerstmann for its accumulation and for his FOMS members it brings a special appeal to sponsorship of the Franklin Mineral Museum dig in the dirt, not to plant, but to explore as the recipient. Transfer of the collection again the crystalline mysteries of Nature. will be effected as soon as suitable space is available to house it. Let us not lose sight of the fact that we are a community, however widespread, dedicated JLB to a great common interest and purpose: the expansion and preservation of knowledge about the world's most remarkable mineral location. ABOUT THE COVER SKETCH Like all great enterprises, this demands the efforts and participation of many. To the Located Sphalerite Occurrences—Franklin Mine extent that we share our knowledge, our time, and our interest with each other and the world, It is suggested that you refer to this hand Franklin lives. -

MINERALS and MINERAL VARIETIES from METAMORPHOSED Mn DEPOSITS of BISTRITA MOUNTAINS, ROMANIA
Acta Mineralogica-Petrographica, Abstract Series 1, Szeged, 2003 MINERALS AND MINERAL VARIETIES FROM METAMORPHOSED Mn DEPOSITS OF BISTRITA MOUNTAINS, ROMANIA HÎRTOPANU, P.1 & SCOTT, P.2 1 Geological Institute of Romania, Caransebeş 1, RO-78344 Bucharest, Romania. E-mail: [email protected] 2 Camborne School of Mines, Redruth, Cornwall, United Kingdom. The Bistrita Mountains belong to the Crystalline Meso- mation of some amphiboles and some pyroxenes into other zoic Zone of the East Carpathians, which consists of super- phases, there are drastical transformations of pyroxenes into posed Variscan and Alpine Nappes, overthrusted eastwards pyroxenoids (johannsenite into rhodonite), pyroxenoids into over the Flysch Zone. The manganese ore is contained by pyroxenoids (pyroxmangite into rhodonite), pyroxmangite Tulghes Group (Tg2 level) of the Variscan Putna Nappe, into manganogrunerite, garnets into garnets (spessartine- situated over the Pietrosu Bistritei Nappe and supporting the calderite into spessartine, spessartine into anisotropic spes- thrusting of the Rebra Nappe. All these Variscan nappes sartine-andradite-grossular), calderite into pyroxmangite- constitute the Alpine Sub-Bucovinian Nappe localised be- magnetite, etc. are the best evidences of continuous variation tween Alpine Infrabucovinian Nappe in the East and the of formation conditions. Alpine Bucovinian Nappe in the West. The Mn ore have a predominant carbonate rather than The mineralogy of Mn metamorphosed deposits from silicate mineralogical composition, which means a great CO2 Bistrita Mts. includes 328 minerals and mineral varieties. fluid control in the carbonation and dehydration processes They may count among the mineralogically the most com- along the many stages of the whole history of the ore and the plex deposits of the world. -

Kinoshitalite (Ba,K)(Mg,Mn,Al)3Si2al2o10(OH)2
Kinoshitalite (Ba; K)(Mg; Mn; Al)3Si2Al2O10(OH)2 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Forms small scales, < 1 mm. Physical Properties: Cleavage: 001 , perfect. Tenacity: Brittle. Hardness = 2.5{3 D(meas.) = 3.30 D(calc.) = 3.33 f g Optical Properties: Semitransparent. Color: Yellow-brown to colorless; light yellow to colorless in thin section. Luster: Vitreous. Optical Class: Biaxial ({). Pleochroism: X = very light yellow to light yellow; Y = Z = light yellow with brownish tinge. Absorption: Y Z > X. ® = 1.619 ¯ = 1.628{1.633 ° = 1.635 ' 2V(meas.) = 23± Cell Data: Space Group: C2=m: a = 5.345(3) b = 9.250(4) c = 10.256(8) ¯ = 99:99(6)± Z = 2 X-ray Powder Pattern: Noda-Tamagawa mine, Japan. 3.37 (100), 2.52 (55), 2.020 (55), 5.05 (50), 10.1 (45), 1.684 (15), 3.16 (5) Chemistry: (1) (2) (1) (2) SiO2 24.58 23.43 BaO 17.85 27.60 TiO2 0.16 Na2O 0.68 0.11 Al2O3 22.06 19.25 K2O 3.30 0.24 Fe2O3 0.71 1.87 F 0.21 + Mn2O3 3.24 H2O 2.90 FeO 0.04 H2O¡ 0.20 MnO 7.38 2.62 H2O 3.50 MgO 16.60 21.95 O = F 0.09 ¡ 2 CaO 0.05 0.05 Total 99.87 100.62 2+ (1) Noda-Tamagawa mine, Japan; corresponds to (Ba0:58K0:35Na0:11Ca0:01)§=1:05(Mg2:06Mn0:52 3+ 3+ Al0:22Mn0:21Fe0:04Ti0:01)§=3:06Si2:05Al1:94O10[(OH)1:62O0:33F0:06]§=2:01: (2) Netra, India; by electron microprobe, total Fe as Fe2O3; corresponding to (Ba0:93K0:03Na0:02Ca0:01)§=0:99 (Mg2:80Mn0:19Fe0:08)§=3:07Si2:01(Al1:94Fe0:05)§=1:99O10(OH)2: Polymorphism & Series: 1M, 2M1 polytypes. -

Characterisation of Ni Silicate-Bearing Minerals by UV-Vis-NIR Spectrscopy
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Bayyareddy, Jagannadha, Frost, Ray,& Dickfos, Marilla (2009) Characterisation of Ni silicate-bearing minerals by UV-vis-NIR spec- trscopy: Effect of Ni substitution in hydrous Ni-Mg silicates. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 71(5), pp. 1762-1768. This file was downloaded from: https://eprints.qut.edu.au/15661/ c Copyright 2009 Elsevier Reproduced in accordance with the copyright policy of the publisher. Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. https://doi.org/10.1016/j.saa.2008.06.030 QUT Digital Repository: http://eprints.qut.edu.au/ Reddy, B. Jagannadha and Frost, Ray L. and Dickfos, Marilla J. (2009) Characterisation of Ni silicate-bearing minerals by UV-Vis-NIR spectroscopy - Effect of Ni substitution in hydrous Ni-Mg silicates . Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 71:pp. 1762-1768. © Copyright 2009 Elsevier 1 2 Characterisation of Ni silicate-bearing minerals by UV-Vis-NIR spectroscopy 3 -Effect of Ni substitution in hydrous Ni-Mg silicates 4 5 B. Jagannadha Reddy, Ray L. Frost •and Marilla J. Dickfos 6 7 Inorganic Materials Research program, School of Physical and Chemical Sciences, 8 Queensland University of Technology, GPO Box 2434, Brisbane Queensland 4001, 9 Australia. -

Leucophoenicite Mn (Sio4)3(OH)2
2+ Leucophoenicite Mn7 (SiO4)3(OH)2 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Crystals rare, typically slender, prismatic, elongated and striated [010], to 8 mm; in isolated grains or granular massive. Twinning: On k 001 , common, contact or interpenetrant twins, lamellar. f g Physical Properties: Cleavage: 001 , imperfect. Tenacity: Brittle. Hardness = 5.5{6 f g D(meas.) = 3.848 D(calc.) = [4.01] Optical Properties: Transparent to translucent. Color: Brown to light purple-red, raspberry-red, deep pink to light pink; rose-red to colorless in thin section. Luster: Vitreous. Optical Class: Biaxial ({). Pleochroism: Faint; rose-red 001 ; colorless 001 . Orientation: k f g ? f g X 001 cleavage. Dispersion: r > v; slight. ® = 1.751(3) ¯ = 1.771(3) ° = 1.782(3) ? f g 2V(meas.) = 74(5)± Cell Data: Space Group: P 21=a: a = 10.842(19) b = 4.826(6) c = 11.324(9) ¯ = 103:93(9)± Z = [2] X-ray Powder Pattern: Franklin, New Jersey, USA. 1.8063 (10), 2.877 (9), 2.684 (8), 4.36 (5), 3.612 (5), 2.365 (5), 2.620 (4) Chemistry: (1) (2) (3) (1) (2) (3) SiO2 26.36 26.7 26.7 CaO 5.67 2.4 2.8 FeO trace 0.3 0.3 Na2O 0.39 MnO 60.63 62.8 64.7 K2O 0.24 ZnO 3.87 0.0 0.0 H2O 2.64 [2.3] [2.8] MgO 0.21 5.5 2.7 Total 100.01 [100.0] [100.0] (1) Franklin, New Jersey, USA; composite of two analyses, corresponding to (Mn5:89Ca0:70Zn0:32 Na0:04Mg0:03K0:01)§=6:99(Si1:01O4)3(OH)2: (2) Kombat mine, Namibia; by electron microprobe, H2O by di®erence; corresponding to (Mn5:98Mg0:92Ca0:29Fe0:02)§=7:21(SiO4)3(OH)1:72: (3) Valsesia-Valtournanche area, Italy; by electron microprobe, H2O by di®erence; corresponding to (Mn6:16Mg0:45Ca0:34Fe0:03)§=6:98(SiO4)3(OH)2:10: Mineral Group: Leucophoenicite group. -

A New Mineral from Mn Ores of the Ushkatyn-III Deposit, 3 Central Kazakhstan and Metamorphic Rocks of the Wanni Glacier, Switzerland 4 5 Oleg S
1 Revision 1 2 Gasparite-(La), La(AsO4), a new mineral from Mn ores of the Ushkatyn-III deposit, 3 Central Kazakhstan and metamorphic rocks of the Wanni glacier, Switzerland 4 5 Oleg S. Vereshchagin*1, Sergey N. Britvin1,6, Elena N. Perova1, Aleksey I. Brusnitsyn1, 6 Yury S. Polekhovsky1, Vladimir V. Shilovskikh2, Vladimir N. Bocharov2, 7 Ate van der Burgt3, Stéphane Cuchet4, and Nicolas Meisser5 8 1Institute of Earth Sciences, St. Petersburg State University, University Emb. 7/9, 199034 St. 9 Petersburg, Russia 10 2Geomodel Centre, St. Petersburg State University, Uliyanovskaya St. 1, 198504 St. 11 Petersburg, Russia 12 3Geertjesweg 39, NL-6706EB Wageningen, The Netherlands 13 4ch. des Bruyeres 14, CH-1007 Lausanne, Switzerland 14 5Musée cantonal de géologie, Université de Lausanne, Anthropole, 1015 Lausanne, 15 Switzerland 16 6Kola Science Center, Russian Academy of Sciences, Fersman Str. 14, 184209 Apatity, 17 Murmansk Region, Russia 18 *E-mail: [email protected] 19 1 20 Abstract 21 Gasparite-(La), La(AsO4), is a new mineral (IMA 2018-079) from Mn ores of the Ushkatyn- 22 III deposit, Central Kazakhstan (type locality) and from alpine fissures in metamorphic rocks 23 of the Wanni glacier, Binn Valley, Switzerland (co-type locality). Gasparite-(La) is named 24 for its dominant lanthanide, according to current nomenclature of rare-earth minerals. 25 Occurrence and parageneses in both localities is distinct: minute isometric grains up to 15 µm 26 in size, associated with fridelite, jacobsite, pennantite, manganhumite series minerals 27 (alleghanyite, sonolite), sarkinite, tilasite and retzian-(La) are typically embedded into 28 calcite-rhodochrosite veinlets (Ushkatyn-III deposit), versus elongated crystals up to 2 mm in 29 size in classical alpine fissures in two-mica gneiss without indicative associated minerals 30 (Wanni glacier). -

The Phase Diversity of Nickel Phyllosilicates from New Caledonia: an Investigation Using Transmission Electron Microscopy (TEM) STUDENT: Brittany A
THE PHASE DIVERSITY OF NICKEL PHYLLOSILICATES FROM NEW CALEDONIA: AN INVESTIGATION USING TRANSMISSION ELECTRON MICROSCOPY (TEM) A THESIS SUBMITTED TO THE GRADUATE SCHOOL IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE MASTER OF SCIENCE BY BRITTANY CYMES DR. KIRSTEN NICHOLSON – ADVISOR BALL STATE UNIVERSITY MUNCIE, INDIANA MAY 2016 ABSTRACT THESIS: The Phase Diversity of Nickel Phyllosilicates from New Caledonia: An Investigation Using Transmission Electron Microscopy (TEM) STUDENT: Brittany A. Cymes DEGREE: Master of Science COLLEGE: Sciences and Humanities DATE: May, 2016 PAGES: 136 New Caledonia is a French territorial island in the Southwest Pacific with an economy heavily dependent upon nickel-mining, being the 5th largest producer worldwide. The nickel deposits result from tropical lateritic weathering of ophiolite units that were emplaced during the late Eocene. The ultramafic units weathered and continue to weather into bright green Ni-rich phyllosilicates colloquially referred to as „garnierite‟. Detailed investigations of „garnierite‟ using transmission electron microscopy (TEM) have been carried out in other locations to characterize important nanoscale features; however, none have been undertaken in New Caledonia. In this investigation, ten samples of „garnierite‟ from New Caledonia were examined with powder X-ray diffraction (XRD) and TEM with energy dispersive spectrometry (EDS). The XRD results show the phyllosilicate material to be comprised of highly disordered talc- and serpentine-like minerals with minor chlorite indicated by significantly broad reflections and two- i dimensional diffraction bands, consistent with published data. TEM analysis, however, revealed new information. Talc-like minerals occur as fluffy aggregates of crystals with lattices with approximately 10 Å d-spacing and significant crystallographic disorder and also as aggregates of larger crystals with more orderly lattices; these two varieties represent the kerolite-pimelite series and the talc- willemseite series, respectively.