Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Syntectonic Mobility of Supergene Nickel Ores of New Caledonia (Southwest Pacific)
Syntectonic mobility of supergene nickel ores of New Caledonia (Southwest Pacific). Evidence from faulted regolith and garnierite veins. Dominique Cluzel, Benoit Vigier To cite this version: Dominique Cluzel, Benoit Vigier. Syntectonic mobility of supergene nickel ores of New Caledonia (Southwest Pacific). Evidence from faulted regolith and garnierite veins.. Resource Geology, Wiley- Blackwell publishing, 2008, 58 (2), pp.161 - 170. 10.1111/j.1751-3928.2008.00053.x. hal-00161201 HAL Id: hal-00161201 https://hal.archives-ouvertes.fr/hal-00161201 Submitted on 10 Jul 2007 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Syntectonic mobility of supergene nickel ores of New Caledonia (Southwest Pacific). Evidence from faulted regolith and garnierite veins. Dominique CLUZEL and Benoit VIGIER Institut des Sciences de la Terre d'Orléans, ISTO, UMR 6113, University of Orleans, BP 6759, 45067 Orléans Cedex 2, France. [email protected] Running title: Syntectonic mobility of supergene nickel ores Abstract Supergene nickel deposits of New Caledonia that have been formed in the Neogene by weathering of obducted ultramafic rocks are tightly controlled by fracture development. The relationship of tropical weathering and tectonic structures, faults and tension gashes, have been investigated in order to determine whether fractures have play a passive role only, as previously thought; or alternatively, if brittle tectonics was acting together with alteration. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Thermal and Infrared Studies of Garnierite from the Soroako Nickeliferous Laterite Deposit, Sulawesi, Indonesia
Indonesian Journal of Geology, Vol. 7 No. 2 June 2012: 77-85 Thermal and Infrared Studies of Garnierite from the Soroako Nickeliferous Laterite Deposit, Sulawesi, Indonesia Analisis Termal dan Inframerah Garnierit dari Endapan Laterit Nikel Saroako, Sulawesi, Indonesia SUFRIADIN1,3*, A. IDRUS1, S. PRAMUMIJOYO1, I W. WARMADA1, I. NUR1,3, A. IMAI2, A.M. IMRAN3, and KAHARUDDIN3 1Department of Geological Engineering, Gadjah Mada University, Yogyakarta 55281, Indonesia 2Department of Earth Science and Technology, Akita University, Akita 010-8512, Japan 3Department of Geological Engineering, Hasanuddin University, Makassar 90245, Indonesia ABSTRACT Mineralogical characterization of some garnierite samples from Soroako have been conducted using X-ray diffraction, thermal analysis, and infrared spectroscopy methods. XRD patterns reveal the samples mainly containing the mixture of kerolite (talc-like phase) and serpentine with minor smectite, sepiolite, and silica. Thermal analyses of garnierite samples indicated by DTA curves are in good agreement with patterns that have been reported in literature. Three endothermic peaks normally occur in the ranges between 58º C and <800º C illustrating three steps of weight losses: adsorbed, bound, and hydroxyl/crystal water. One additional weight loss in low temperature region of sepiolite is corresponding to the lost of zeolitic water. Infrared spectra appeared in 3800 - 3200 cm-1 region generally exhibit broad absorption bands, indicating low crystallinities of studied samples and can be assigned to the presence of hydroxyl group bonded to octahedral coordina- tion mainly Mg atom. The bands observed at 1660 cm-1, 1639 cm-1, 1637 cm-1, and 1633 cm-1 in all samples indicate water molecules. FTIR spectra displaying the strong bands at 1045 cm-1, 1038 cm-1, and 1036 cm-1 could be related to the presence of Si-O-Si bonds linking to tetrahedral coordination. -

DOGAMI MP-20, Investigations of Nickel in Oregon
0 C\1 a: w a.. <( a.. en ::::> 0 w z <( __j __j w () en � INVESTIGATIONS OF NICKEL IN OREGON STATE OF OREGON DEPARTMENT OF GEOL.OGY AND MINERAL. IN OUSTRIES DONAL.D .A HUL.L. STATE GEOLOGIST 1978 STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL INDUSTRIES 1069 State Office Building, Portland, Oregon 97201 MISCELLANEOUS PAPER 20 INVESTIGATIONS OF NICKEL IN OREGON Len Ramp, Resident Geologist Grants Pass Field Office Oregon Department of Geology and Mineral Industries Conducted in conformance with ORS 516.030 . •. 5 1978 GOVERNING BOARD Leeanne MacColl, Chairperson, Portland Talent Robert W. Doty STATE GEOLOGIST John Schwabe Portland Donald A. Hull CONTENTS INTRODUCTION -- - ---- -- -- --- Purpose and Scope of this Report Acknowledgments U.S. Nickel Industry GEOLOGY OF LATERITE DEPOSITS - -- - 3 Previous Work - - - - --- 3 Ultramafic Rocks - ----- --- 3 Composition - - -------- - 3 Distribution ------ - - - 3 Structure - 3 Geochemistry of Nickel ---- 4 Chemical Weathering of Peridotite - - 4 The soi I profile ------- 5 M i nero I ogy -- - ----- 5 Prospecting Guides and Techniques- - 6 OTHER TYPES OF NICKEL DEPOSITS - - 7 Nickel Sulfide Deposits- - - - - - 7 Deposits in Oregon 7 Other areas --- 8 Prospecting techniques 8 Silica-Carbonate Deposits - -- 8 DISTRIBUTION OF LATERITE DEPOSITS - ------ 9 Nickel Mountain Deposits - - ------ --------- 9 Location --------------- --- 9 Geology - ------- ----- 11 Ore deposits ----------- - -- 11 Soil mineralogy - ------- 12 Structure --- ---- ---- 13 Mining and metallurgy ------------ ---- 13 Production- -
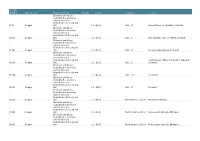
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

Swelling Capacity of Mixed Talc-Like/Stevensite Layers in White/Green Clay
This is a preprint, the final version is subject to change, of the American Mineralogist (MSA) Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2020-6984 1 1 Plagcheck: no concerns 2 Tables?: 3 small 3 Word Count: ~9,100 4 Prod notes: make sure tables in file before RE 5 6 7 8 Swelling capacity of mixed talc-like/stevensite layers in white/green clay 9 infillings (‘deweylite’/‘garnierite’) from serpentine veins of faulted 10 peridotites, New Caledonia 11 REVISION 2 12 Lionel FONTENEAU 1, Laurent CANER 2*, Sabine PETIT 2, Farid JUILLOT 3, Florian 13 PLOQUIN 3, Emmanuel FRITSCH 3 14 15 1Corescan Pty Ltd, 1/127 Grandstand Road, 6104 Ascot, WA, Australia 16 2 Université de Poitiers, Institut de Chimie des Milieux et Matériaux de Poitiers, IC2MP UMR 17 7285 CNRS, 5 rue Albert Turpain, TSA51106, 86073 Poitiers cedex 9, France 18 * Corresponding author, e-mail: [email protected] 19 3 Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie (IMPMC), Sorbonne 20 Universités – Université Pierre et Marie Curie UPMC, UMR CNRS 7590, Museum National 21 d’Histoire Naturelle, UMR IRD 206, 101 Promenade Roger Laroque, Anse Vata, 98848, 22 Nouméa, New Caledonia 23 24 25 Abstract: White (Mg-rich) and green (Ni-rich) clay infillings (‘deweylite’/‘garnierite’) found 26 in serpentine veins of faulted peridotite formations from New Caledonia consist of an intimate 27 mixture of fine-grained and poorly ordered 1:1 and 2:1 layer silicates, commonly referred to 28 as non-expandable serpentine-like (SL) and talc-like (TL) minerals. -

Characterisation of Ni Silicate-Bearing Minerals by UV-Vis-NIR Spectrscopy
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Bayyareddy, Jagannadha, Frost, Ray,& Dickfos, Marilla (2009) Characterisation of Ni silicate-bearing minerals by UV-vis-NIR spec- trscopy: Effect of Ni substitution in hydrous Ni-Mg silicates. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 71(5), pp. 1762-1768. This file was downloaded from: https://eprints.qut.edu.au/15661/ c Copyright 2009 Elsevier Reproduced in accordance with the copyright policy of the publisher. Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. https://doi.org/10.1016/j.saa.2008.06.030 QUT Digital Repository: http://eprints.qut.edu.au/ Reddy, B. Jagannadha and Frost, Ray L. and Dickfos, Marilla J. (2009) Characterisation of Ni silicate-bearing minerals by UV-Vis-NIR spectroscopy - Effect of Ni substitution in hydrous Ni-Mg silicates . Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 71:pp. 1762-1768. © Copyright 2009 Elsevier 1 2 Characterisation of Ni silicate-bearing minerals by UV-Vis-NIR spectroscopy 3 -Effect of Ni substitution in hydrous Ni-Mg silicates 4 5 B. Jagannadha Reddy, Ray L. Frost •and Marilla J. Dickfos 6 7 Inorganic Materials Research program, School of Physical and Chemical Sciences, 8 Queensland University of Technology, GPO Box 2434, Brisbane Queensland 4001, 9 Australia. -

The Phase Diversity of Nickel Phyllosilicates from New Caledonia: an Investigation Using Transmission Electron Microscopy (TEM) STUDENT: Brittany A
THE PHASE DIVERSITY OF NICKEL PHYLLOSILICATES FROM NEW CALEDONIA: AN INVESTIGATION USING TRANSMISSION ELECTRON MICROSCOPY (TEM) A THESIS SUBMITTED TO THE GRADUATE SCHOOL IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE MASTER OF SCIENCE BY BRITTANY CYMES DR. KIRSTEN NICHOLSON – ADVISOR BALL STATE UNIVERSITY MUNCIE, INDIANA MAY 2016 ABSTRACT THESIS: The Phase Diversity of Nickel Phyllosilicates from New Caledonia: An Investigation Using Transmission Electron Microscopy (TEM) STUDENT: Brittany A. Cymes DEGREE: Master of Science COLLEGE: Sciences and Humanities DATE: May, 2016 PAGES: 136 New Caledonia is a French territorial island in the Southwest Pacific with an economy heavily dependent upon nickel-mining, being the 5th largest producer worldwide. The nickel deposits result from tropical lateritic weathering of ophiolite units that were emplaced during the late Eocene. The ultramafic units weathered and continue to weather into bright green Ni-rich phyllosilicates colloquially referred to as „garnierite‟. Detailed investigations of „garnierite‟ using transmission electron microscopy (TEM) have been carried out in other locations to characterize important nanoscale features; however, none have been undertaken in New Caledonia. In this investigation, ten samples of „garnierite‟ from New Caledonia were examined with powder X-ray diffraction (XRD) and TEM with energy dispersive spectrometry (EDS). The XRD results show the phyllosilicate material to be comprised of highly disordered talc- and serpentine-like minerals with minor chlorite indicated by significantly broad reflections and two- i dimensional diffraction bands, consistent with published data. TEM analysis, however, revealed new information. Talc-like minerals occur as fluffy aggregates of crystals with lattices with approximately 10 Å d-spacing and significant crystallographic disorder and also as aggregates of larger crystals with more orderly lattices; these two varieties represent the kerolite-pimelite series and the talc- willemseite series, respectively. -

Talc- and Serpentine-Like “Garnierites” from Falcondo Ni
macla nº 15 . septiembre 2011 revista de la sociedad española de mineralogía 197 Talc- and Serpentine-like “Garnierites” from Falcondo Ni-laterite Deposit (Dominican Republic): a HRTEM approach / CRISTINA VILLANOVA-DE-BENAVENT (1,*), FERNANDO NIETO (2), JOAQUÍN A. PROENZA (1), SALVADOR GALÍ (1) (1) Departament de Cristal·lografia, Mineralogia i Dipòsits Minerals. Facultat de Geologia. Universitat de Barcelona. C/ Martí i Franquès s/n. 08028 Barcelona, Catalunya (España) (2) Departamento de Mineralogía y Petrología e Instituto Andaluz de Ciencias de la Tierra. Universidad de Granada-CSIC. Campus de • Fuerteventura s/n. 18002 Granada (España) INTRODUCTION. from Falcondo Ni-laterite deposit. These “garnierites” mainly occur as mm-cm results are compared to those previously vein fillings in fractures, but also as “Garnierites” represent significant Ni ore obtained through powder XRD and EMP coatings, boxworks and different kinds minerals in the lower horizons of many (CCiT, Universitat de Barcelona). of breccias, within the saprolite horizon Ni-laterite deposits worldwide (e.g. or near the unweathered peridotite, Freyssinet et al., 2005). They consist of Preliminary data allows to classify the close to the base of the lateritic profile. a green, fine-grained mixture of hydrous sampled “garnierites” into two groups, Ni-bearing magnesium phyllosilicates, which display two well distinguishable METHODOLOGY. including serpentine, talc, sepiolite, greenish colours in hand specimen: smectite and chlorite (e.g. Brindley and A representative sample, containing Hang, 1973; Springer, 1974; Brindley et • Bluish bright-green “garnierites” strongly serpentinized peridotite al., 1979). Thus, “garnierite” is a general display colloform textures under the (saprolite) cross-cut by talc-like and descriptive term and is not recognized optical microscope. -

The Journal of R
The Journal of r Volumemmoie 28 Noemmoi. emmoi2 Apriol 200 2 o Notes from the Laboratory Chocolate- brown Opal Inclutsfbns in Gemstones Natural Amethyst hi1 (iemmoiotiuMl Asstvi.it inn .nij C iem Tcstiiv* I .ahoivitoi'v oi Crrot Britain Gemmological Association and Gem Testing Laboratory of Great Britain 27 Greville Street, London ECIN 8TN Tel: 020 7404 3334 Fax: 020 7404 8843 e-mail: [email protected] Website: www.gagtl.ac.uk President: Professor AT. Collins Vice-Presidents: N. W. Deeks, AE. Farn, RA Howie, D.G. Kent, RK. Mitchell Honorary Fellows: Chen Zhonghui, RA Howie, RT. Liddicoat [nr, K. Nassau Honorary Life Members: H. Bank, D.J. Callaghan, E.A [obbins, H. Tillander Council of Management: T.J. Davidson, RR Harding, 1. Mercer, J. Monnickendam, M.I. O'Donoghue, E. Stern, 1. Thomson, v.P. Watson Members' Council: AJ. Allnutt, S. Burgoyne, P. Dwyer-Hickey, S.A. Everitt, J. Greatwood, B. Jackson, L. Music, J.B. Nelson, P.G. Read, P.J. Wates, C.H. Winter Branch Chairmen: Midlands - G.M. Green, North West - D. M. Brady, Scottish - B. Jackson, South West - RM. Slater Examiners: AJ. Allnutt, M.Sc., PhD., FGA, L. Bartlett, B.5c., M.Phil., FGA, DGA, S. Coelho, B.5c., FGA, DGA, Prof. AT. Collins, B.Sc., Ph.D, A.G. Good, FGA, DGA, I. Greatwood, FGA, G.M. Howe, FGA, DGA, S. Hue Williams MA, FGA, DGA, B. Jackson, FGA, DGA, G.B. Jones, B.5c., Ph.D., FGA, Li Li Ping, FGA, DGA, M. Newton, B.Sc.,D.Phil., c.I.E. Oldershaw, B.Sc. -

The Picking Table Volume 8, No. 1
THE PICKING TABLE FRANKLIN OGDENSBURG MINSHALOGICAL SOCIETY, INC, P. 0. BOJC 146 FRANKLIN, H.J., 07416 VOLUME VIII FEBRUARY 196? NUMBER 1 The contents of The Picking Table are licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. CLUB PROGRAM - SPRING 1967 All meetings will be held at the Hardyston School, intersection of Routes #23 and #517, Franklin, N. J. Pre meeting activities start at 1:00 P.A. Speaker will be announced at 2:30 P.M. Sunday, Field trip, 9=00 A..-I. to Noon - March 19th. Buckwheat Dump, Franklin, N.J. Meeting, 2:30 P.M. Speaker, Paul Desautels Subject - Blood Relatives Among the Minerals. Saturday, Field trip, 9:00 A.M. to Noon - April 15th Buckwheat Dump, Franklin, N.J. Meeting, 2:30 P.M. Speaker - Dr. Paul Moore. Subject - The Mineralogy of Langban, Sweden. Saturday, Field trip, 9:00 A.M. to Noon - Open Cuts, May 20th Sterling Hill Mine, Ogdensburg, H. J. Meeting, 2:30 P.M. Speaker - Dr. Clifford Frondel Subject - Franklin Minerals, New and Old Saturday, Field trio, 9:00 A.M. to Noon - June 17th Farber Quarry, Cork Hill Road, Franklin, ..J, Meeting, 2:30 P.JL Speaker - Robert Metsger Subject - The Geology of Sterling Hill. Special Events April 22/23 1967 Earth Science and Gem Show Mineralogical Society of Pennsylvania, Route 30, Lancaster, Pa. May 6/7th 3rd Annual Mineral and Gem Show Matawan Mineralogical Society, Matawan Regional High School, Matawan, ft. June 29/July 2nd 1967 National Gem and Mineral Show, Eastern Federation, Washington Hilton Hotel, Washington, D.C. -

Thermal Annealing and Phase Transformation of Serpentine- Like Garnierite
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 8 January 2021 doi:10.20944/preprints202101.0154.v1 Article Thermal Annealing and Phase Transformation of Serpentine- like Garnierite Arun Kumar1,2, Michele Cassetta1, Marco Giarola3, Marco Zanatta4, Monique Le Guen5, Gian Domenico Soraru6, Gino Mariotto1,* 1Department of Computer Science, University of Verona, 37134, Verona, Italy 2CNR-Institute for Microelectronics and Microsystems, Agrate Brianza, 20864, Agrate, Italy 3Centro Piattaforme Tecnologiche (CPT), University of Verona, 37134, Verona, Italy 4Department of Physics, University of Trento, 38123, Povo, Trento, Italy 5Mining Division, ERAMET, 78190, Trappes, France 6Department of Industrial Engineering, University of Trento, 38123, Povo, Trento, Italy *Correspondence: [email protected] Abstract: This study deals with vibrational and crystallographic aspects of the thermally induced transformation of serpentine-like garnierite into quartz, forsterite, and enstatite occurring at about 620 °C. Powder specimens of garnierite have been annealed in static air between room temperature and 1000 °C. The resulting products from the transformations detected based on thermogravimetric and differential thermal analysis, have been extensively characterized via microRaman spectros- copy, and X-ray diffraction. Our study shows that serpentine-like garnierite consists of a mixture of different mineral species. Furthermore, these garnierites and their composition can provide details based on the mineralogy and the crystalline phases resulting from the thermal treatment. Keywords: Garnierite, Phase transformation, TGA/DSC, XRD, micro Raman spectroscopy 1. Introduction In 1863, Jules Garnier discovered a nickel ore in New Caledonia, a magnesium-based nickel silicate later named as Garnierite [1]. Garnierite is mined from laterite deposits, and it is formed by weathering and secondary mineralization of igneous ultra-mafic rocks.